Arrange the metals Ca, Al, Mg and Zn in decreasing order of their reactivities :
A
Mg>Ca>Al>Zn
B
Ca>Mg>Al>Zn
C
Mg>Ca>Zn>Al
D
Ca>Mg>Zn>Al
Text Solution
The correct Answer is:B
Ca>Mg>Al>Zn
Metals have been arranged based on their relative reactivities in a series,
|
Similar Questions
Knowledge Check
Arrange the elements Zn, Sn, Ca and Al in the increasing order of their reactivity for replacing Cu from CuSO4 solution:
AZn>Sn>Ca>AlBCa>Al>Zn>SnCCa>Al>Sn>ZnDCa>Sn>Zn>AlArrange in decreasing order of reactivity with HCl :
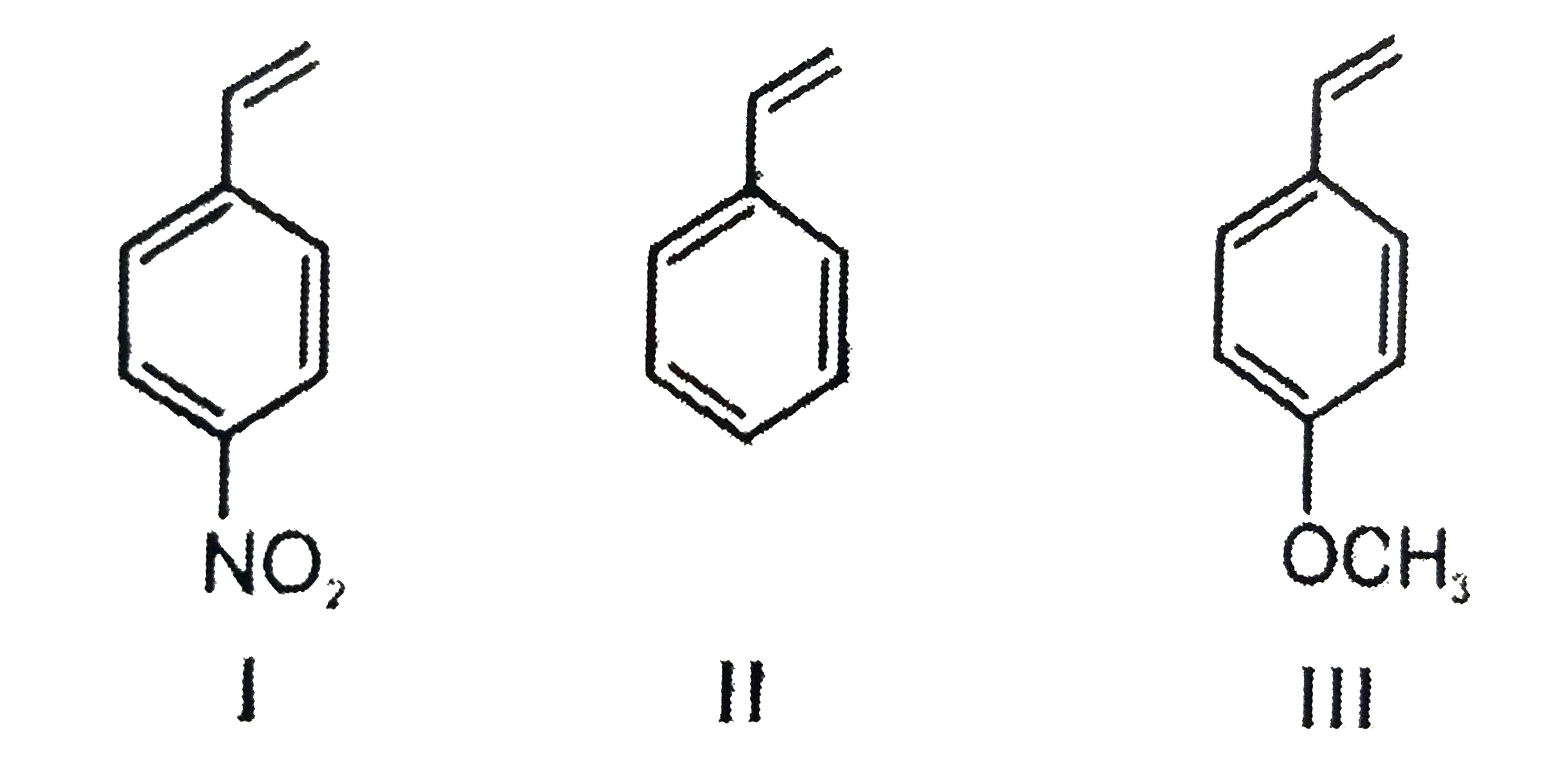 AII gt III gt IBIII gt II gt ICIII gt I gt IIDnone of the above
AII gt III gt IBIII gt II gt ICIII gt I gt IIDnone of the above
Arrange the elements: B, Al, Mg and K in the decreasing order of metallic character.
View SolutionArrange the following metals in the decreasing order of chemical reactivity : Cu, Mg , Fe,Ca,Zn
View SolutionArrange the following metals in the decreasing order of chemical reactivity :
Cu, Mg, Fe, Ca, Zn, Na.
View SolutionArrange the following metals in decreasing order of their reactivity.
Fe,Zn,Na,Cu,Ag
View SolutionHarish took metal samples of Fe,Zn,Al,Cu and Pb in separate test tube. And added hydrochloric acid in each of them.
(a) Arrange the metals in the decreasing order of their reactivity.
View SolutionArrange Be,Mg,Ca,Sr and Ba in increasing order of reactivity.
View Solution
