Will CCl4 give white precipitate of AgCl on heating it with silver nitrate? Give reason for your answer.
Cl4 will not give a white precipitate of AgCl when it is treated with a solution of silver nitrate. It is because Cl4 is a covalent compound and hence does not ionise to give Cl ions, which is the essential requirements for the AgNO3 test.
Topper's Solved these Questions
- PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISExercise:Linked Comprehension Type
Explore 25 Videos
- PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISExercise:Multiple Correct Answers Type
Explore 10 Videos
- PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISExercise:Subjective Type
Explore 11 Videos
- PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRYBook:CENGAGE CHEMISTRY ENGLISHChapter:PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRYExercise:Exercises (Archives )Subjective
Explore 4 Videos
- REDOX REACTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:REDOX REACTIONSExercise:Archives (Integers)
Explore 1 Video
Similar Questions
Qualitative analysis of organic compounds is performed by Lassaigne's test by fusion with metallic sodium, by which the covalent compounds are converted into ionic compounds. Extra elements like N,S,P and halogens are detected by their usual tests.
Q. Which of the following compounds will give positive lassaigne's test for nitrogen
View SolutionWhich of the following statements is/are correct?
View SolutionWhen 100 ml of O2−O3 mixture was passed through turpentine oil, there was reduction of volume by 20 ml. If 100 ml of such a mixture is heated, what will be the increase in the volume?
View SolutionArrange the following in the given order
(a) Decreasing ionic size, Mg2+,O2−,Na⊕,F⊕
(b) Increasing bond length F2,N2,Cl2O2
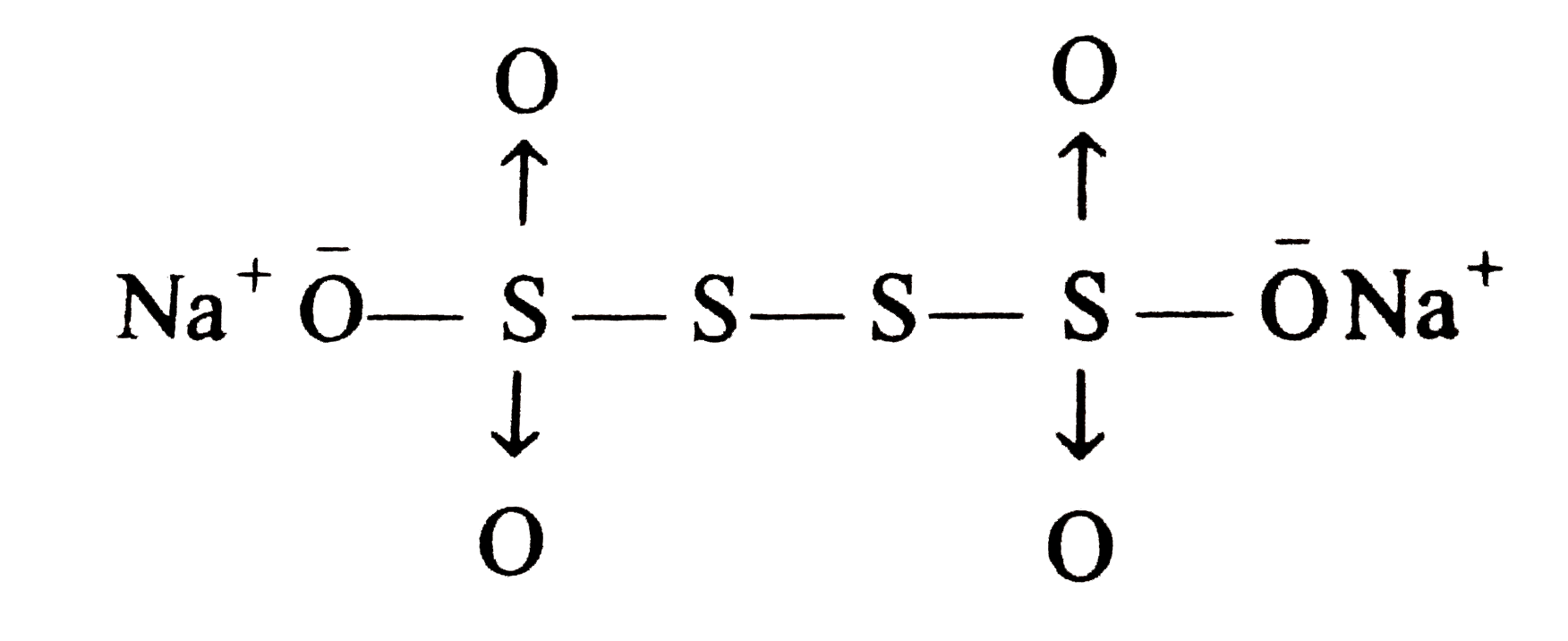
View SolutionThe difference in the oxidation numbers of the two types of sulphur atoms in Na2S4O6 is:
View SolutionWill CCl4 give white precipitate of AgCl on heating with nitrate? Give reason for your answer
View SolutionStatement-1: Orthophosphate gives yellow precipitate with silver nitrate solution. Statement-2: Metaphosphate gives white precipitate with silver nitrate.
View SolutionWill CCl4 give white precipitate of AgCl on heating with nitrate? Give reason for your answer
View Solutionक्या CCl4 सिल्वर नाइट्रेट के साथ गर्म करने पर AgCl का सफेद अवक्षेप देगा?
View SolutionWill CCI4 give white precipitate of AgCI on heating it with silver nitrate? Give reason for your answer.
View SolutionWill CCI4 give white precipitate of AgCI on heating it with silver nitrate solution? Give reason for your answer.
View Solutionअमोनियाक्रत सिल्वर नाइट्रेट सुगमता से सफेद अवक्षेप देता है
View SolutionWill C CI_(4) give white precipitate of AgCl on heating it with silver nitrate? Give reason for your answer.
View SolutionWill CCl4 give white precipitate of AgCl on heating it with silver nitrate? Give reason for your answer.
View SolutionWill CCl4 give white precipitate of AgCl on heating with nitrate? Give reason for your answer
View SolutionAn aqueous solution of silver nitrate gives a white precipitate with
View Solution
