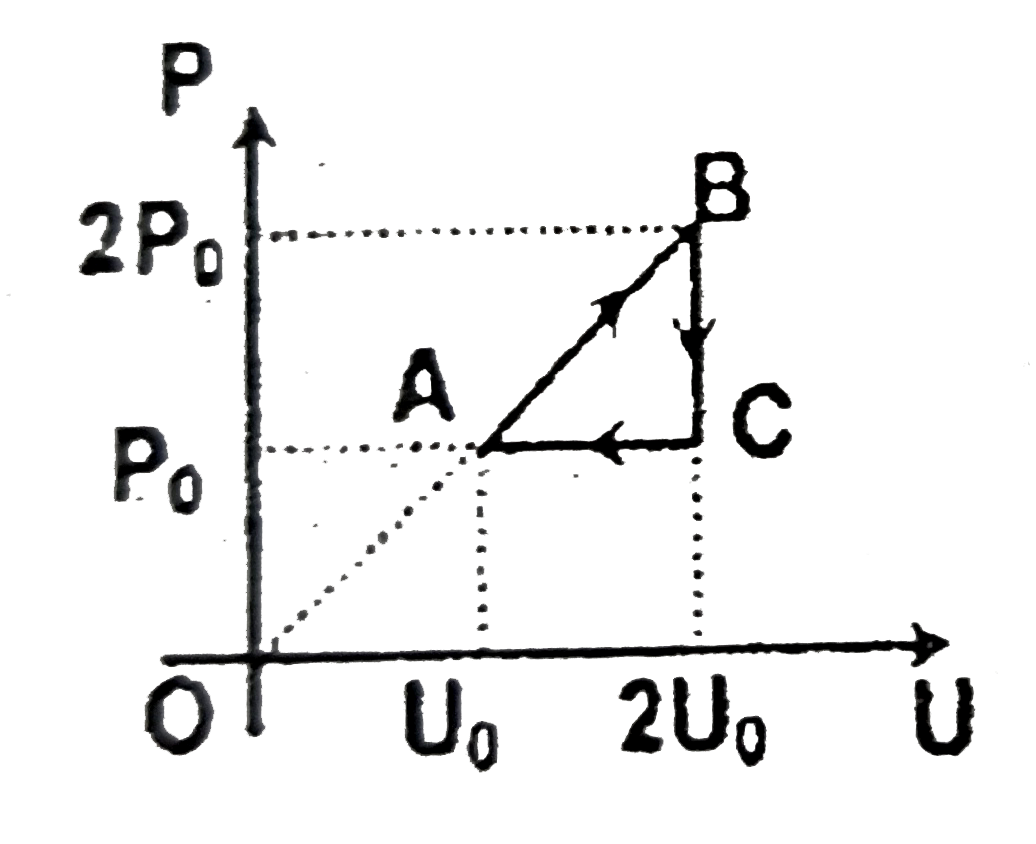
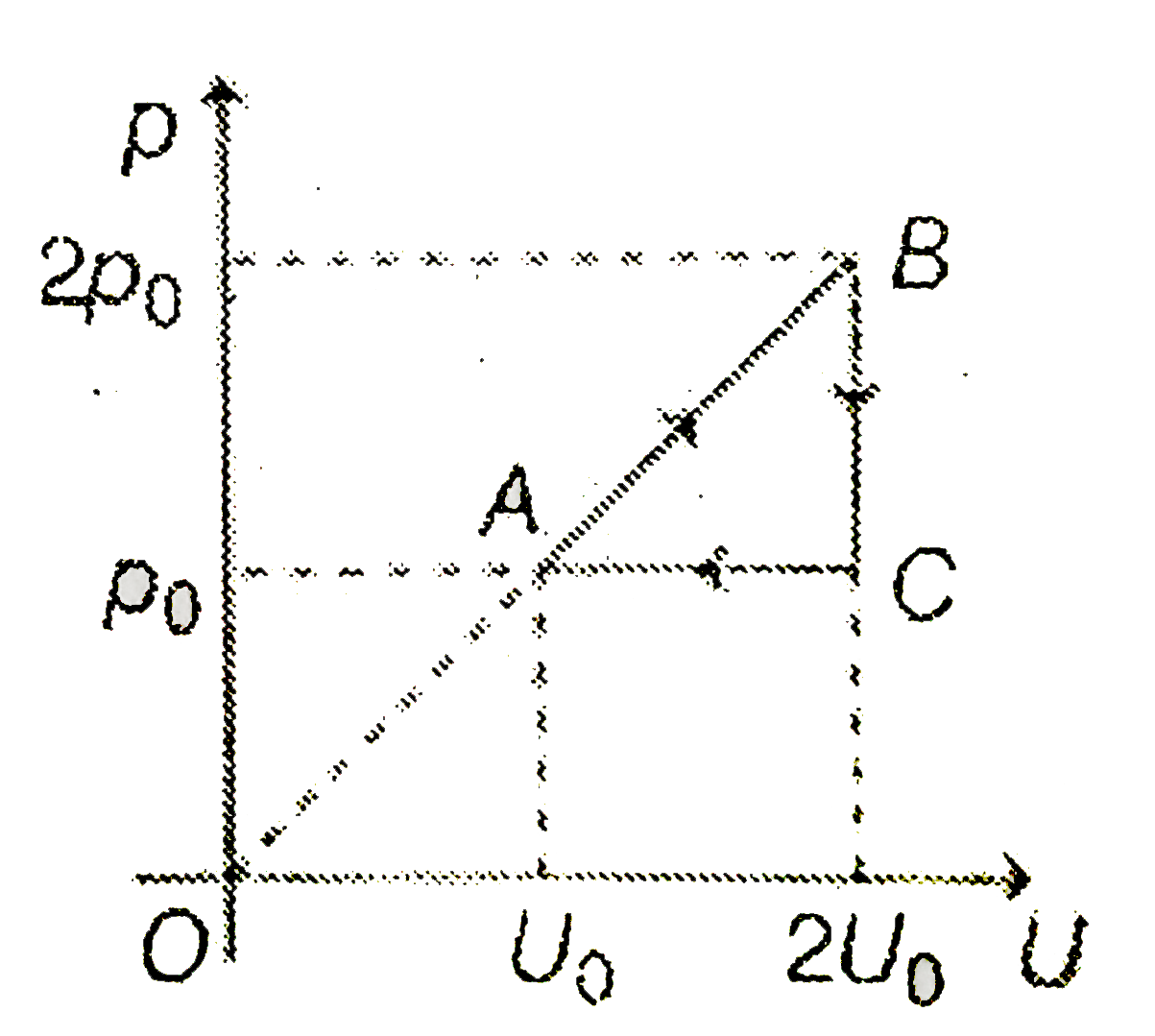
One mole of an ideal gas in initial state A undergoes a cyclic process ABCA, as shown in the figure. Its pressure at A is P0. Choose the correct option (s) from the following
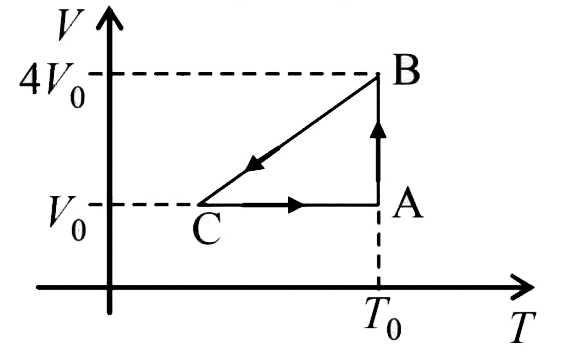
Internal energies at A and B are the same
Work done by the gas in process AB is P0V0(ln4)
Pressure at C is P0/4
Temperature at C is T0/4
The correct Answer is:A, B
Process AB is isothermal. Temperature at A and B is same, so internal energy at A and B is same.
ΔWAB=nRT0ln(VB/VA)
For point A:P0V0=nRT0
So ΔWAB=P0V0ln(4V0/V0)=P0V0ln(4)
Nothing can be said about pressure and temperature at C .
Topper's Solved these Questions
- ARCHIVES 1 VOLUME 6Book:CENGAGE PHYSICS ENGLISHChapter:ARCHIVES 1 VOLUME 6Exercise:Assertion-Reasoning
Explore 1 Video
- ARCHIVES 1 VOLUME 6Book:CENGAGE PHYSICS ENGLISHChapter:ARCHIVES 1 VOLUME 6Exercise:Comprehension
Explore 3 Videos
- ARCHIVES 1 VOLUME 6Book:CENGAGE PHYSICS ENGLISHChapter:ARCHIVES 1 VOLUME 6Exercise:Single Correct
Explore 54 Videos
- ARCHIVES 2 VOLUME 6Book:CENGAGE PHYSICS ENGLISHChapter:ARCHIVES 2 VOLUME 6Exercise:Integer
Explore 4 Videos
Similar Questions
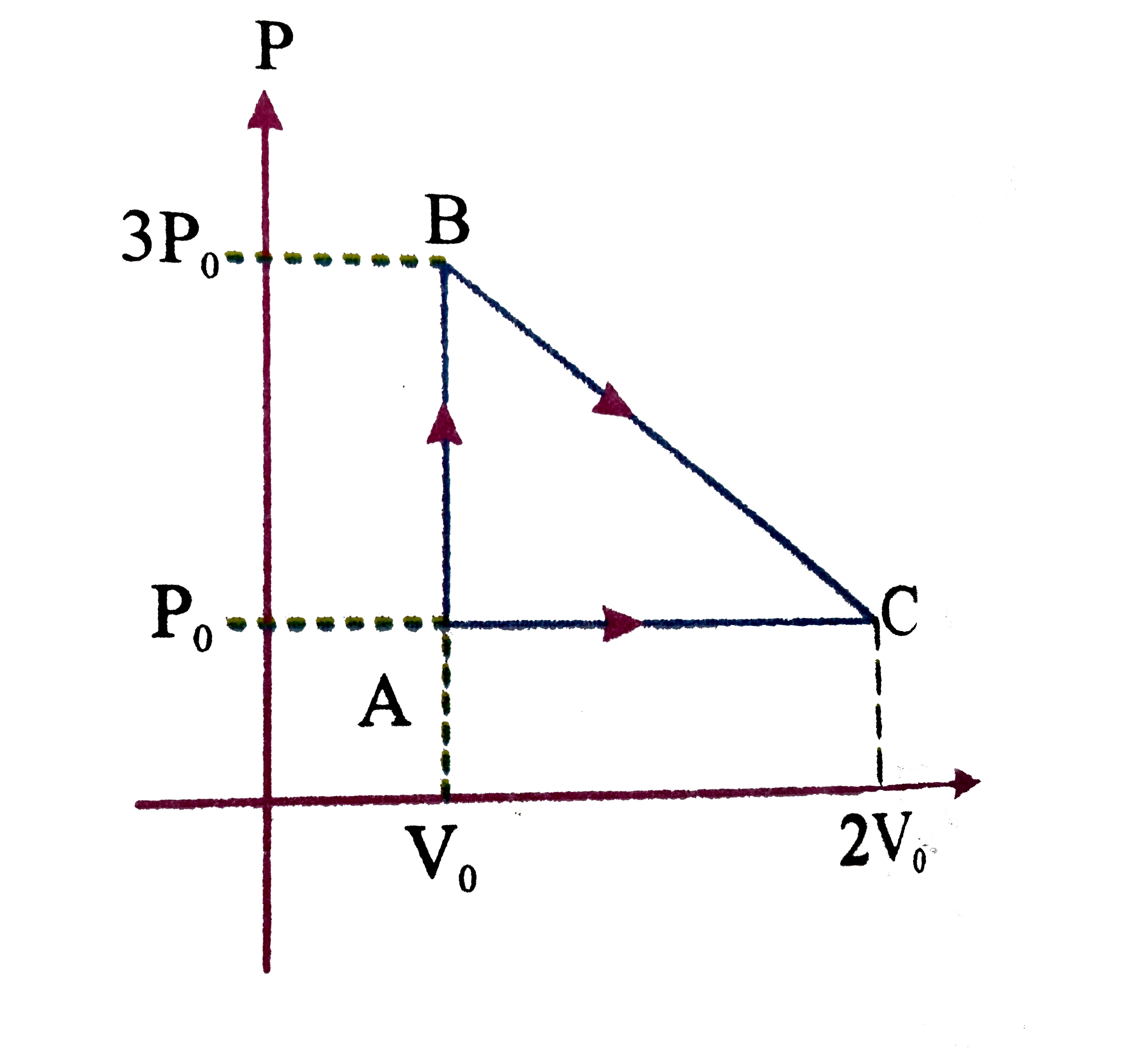
One mole of an ideal monoatomatic gas is taken round the cylic process ABCA as shown Then
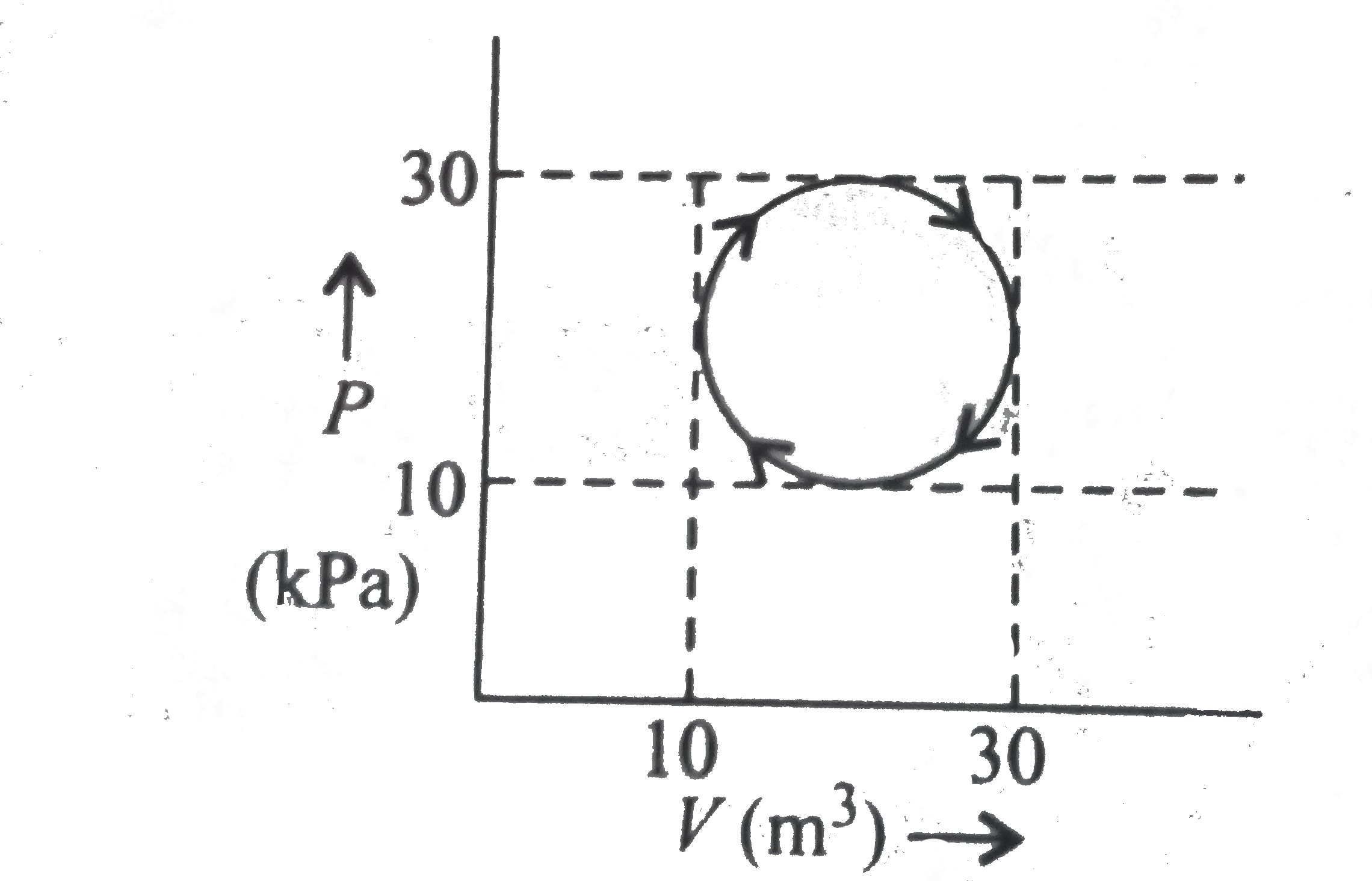
View SolutionWhich one is not correct for a cyclic process as shown in the figure ?
View SolutionAn ideal gas undergoes the cyclic process shown in a graph below :
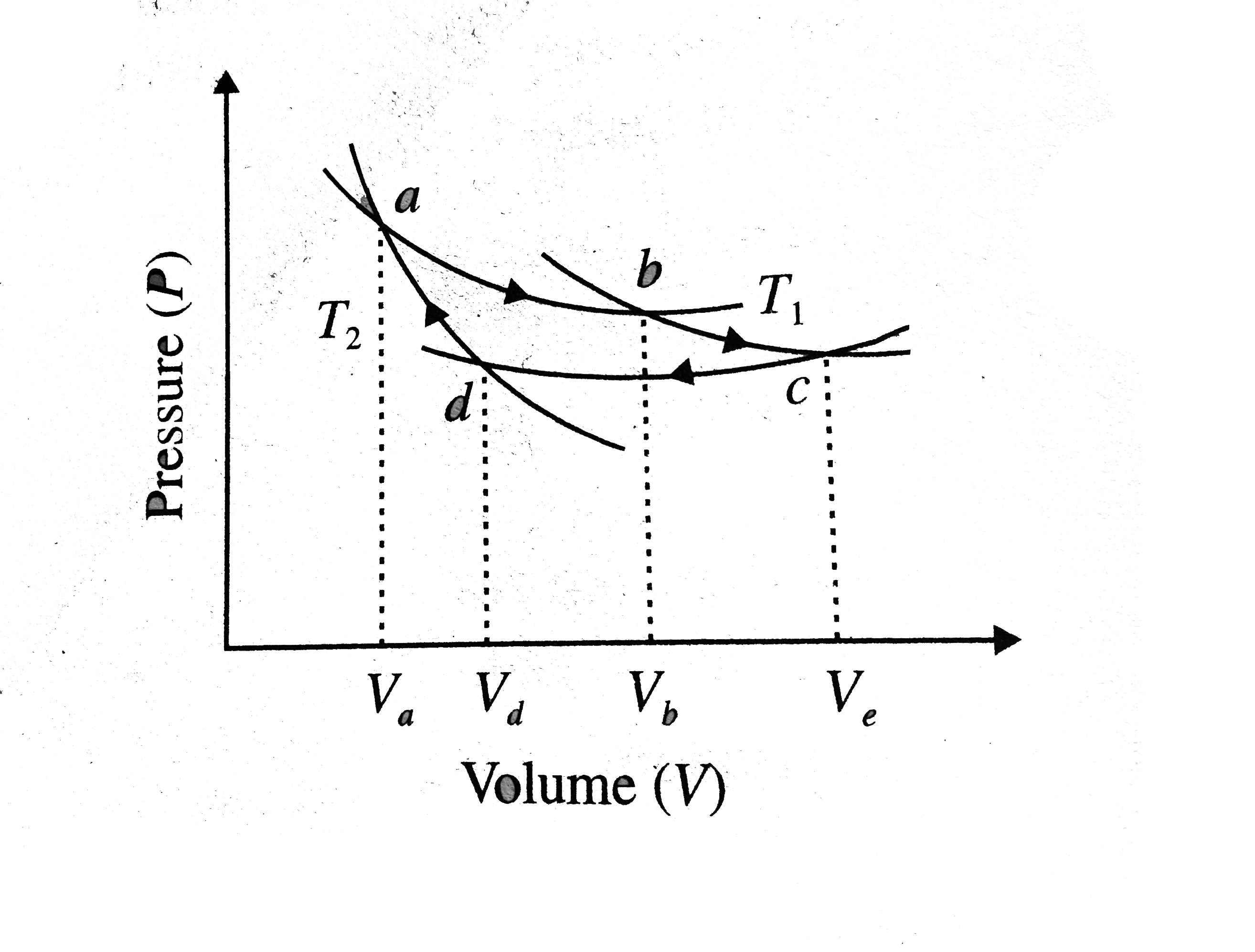
View SolutionAn ideal monatomic gas undergoes a cyclic process ABCA as shown in the figure. Graph of pressure versus volume of the cyclic process ABCA is
View SolutionOne mole of an ideal mono-atomic gas is taken round cyclic process ABC as shown in figure below. Calculate work done
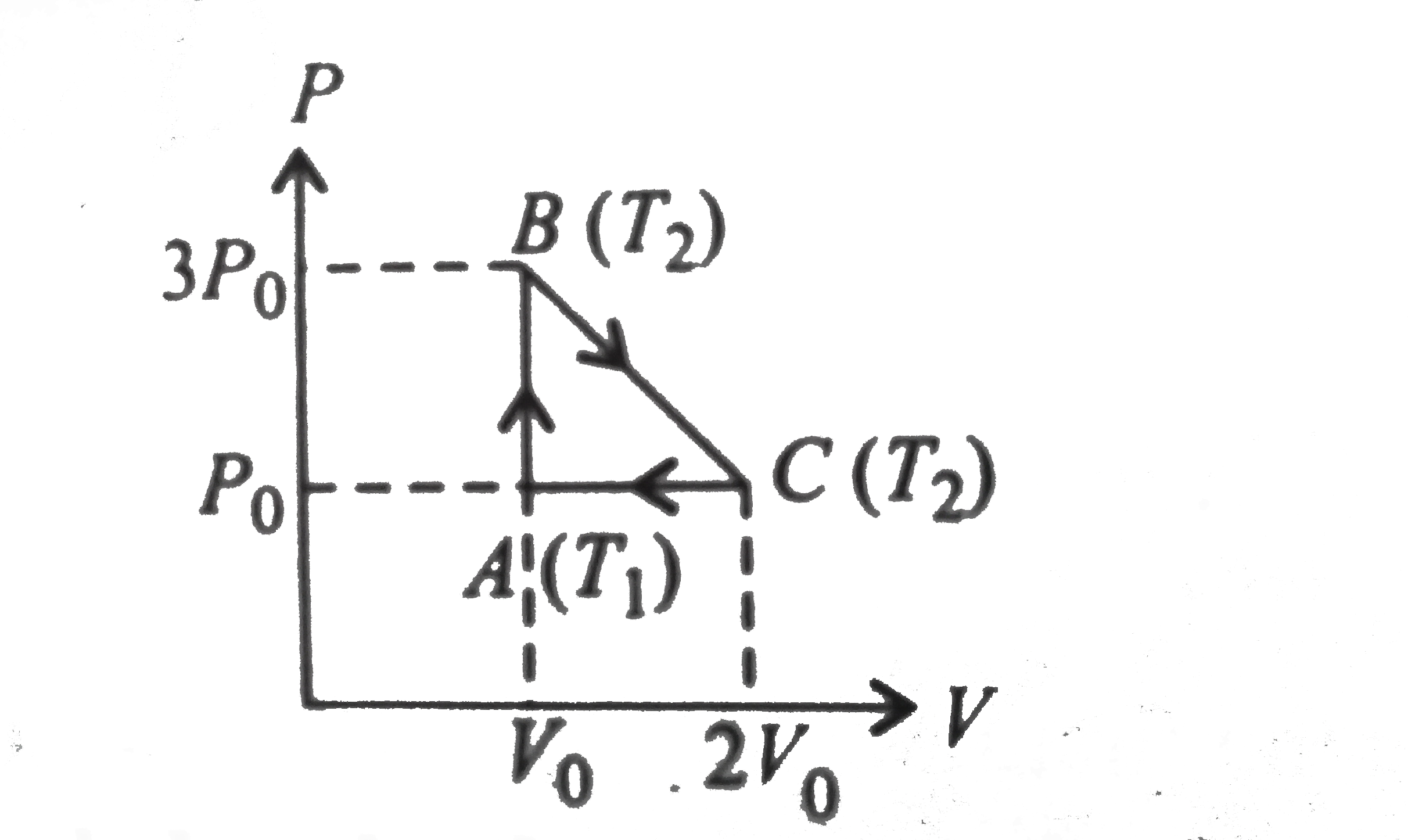
View SolutionOne mole of an ideal mono-atomic gas is taken round cyclic process ABC as shown in figure below. Calculate work done
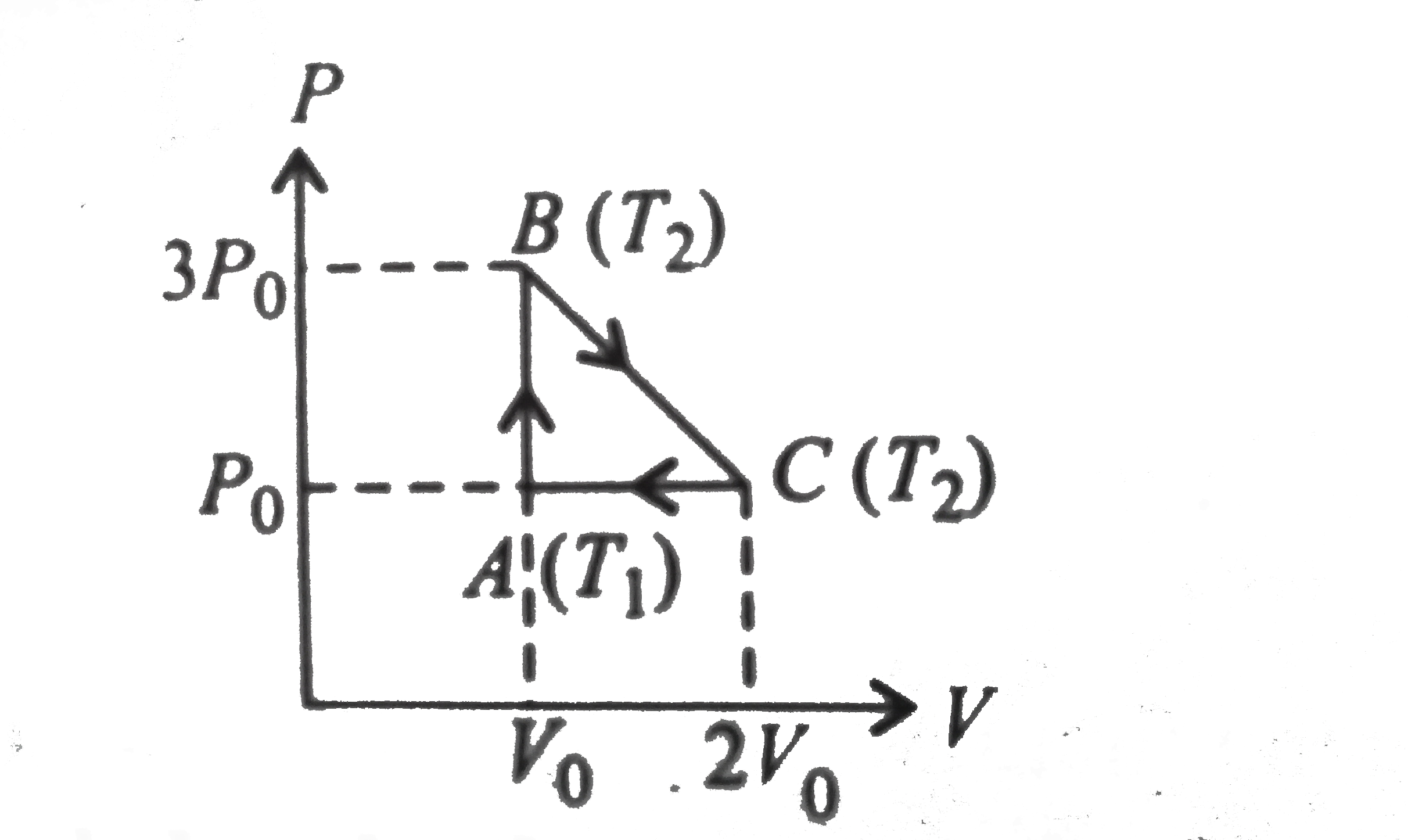
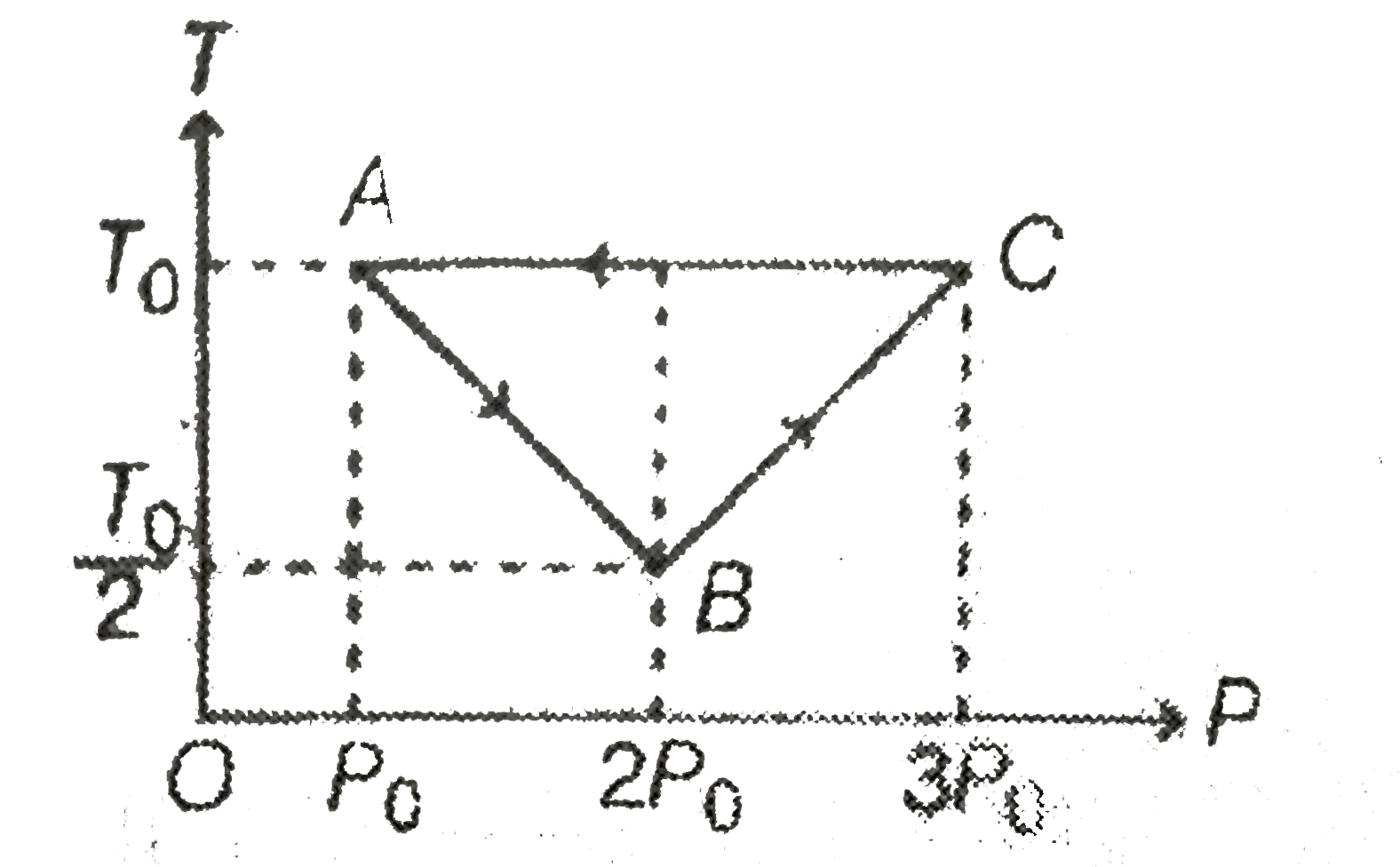
View SolutionOne mole of an ideal monoatomic gas is taken through a cyclic process as shown. Choose the correct option(s).
View Solution2 moles of an ideal monoatomic gas undergoes a cyclic process ABCA as shown in the figure. R is the univers l gas constant. (given ln 2=0.693) Answer the following questions
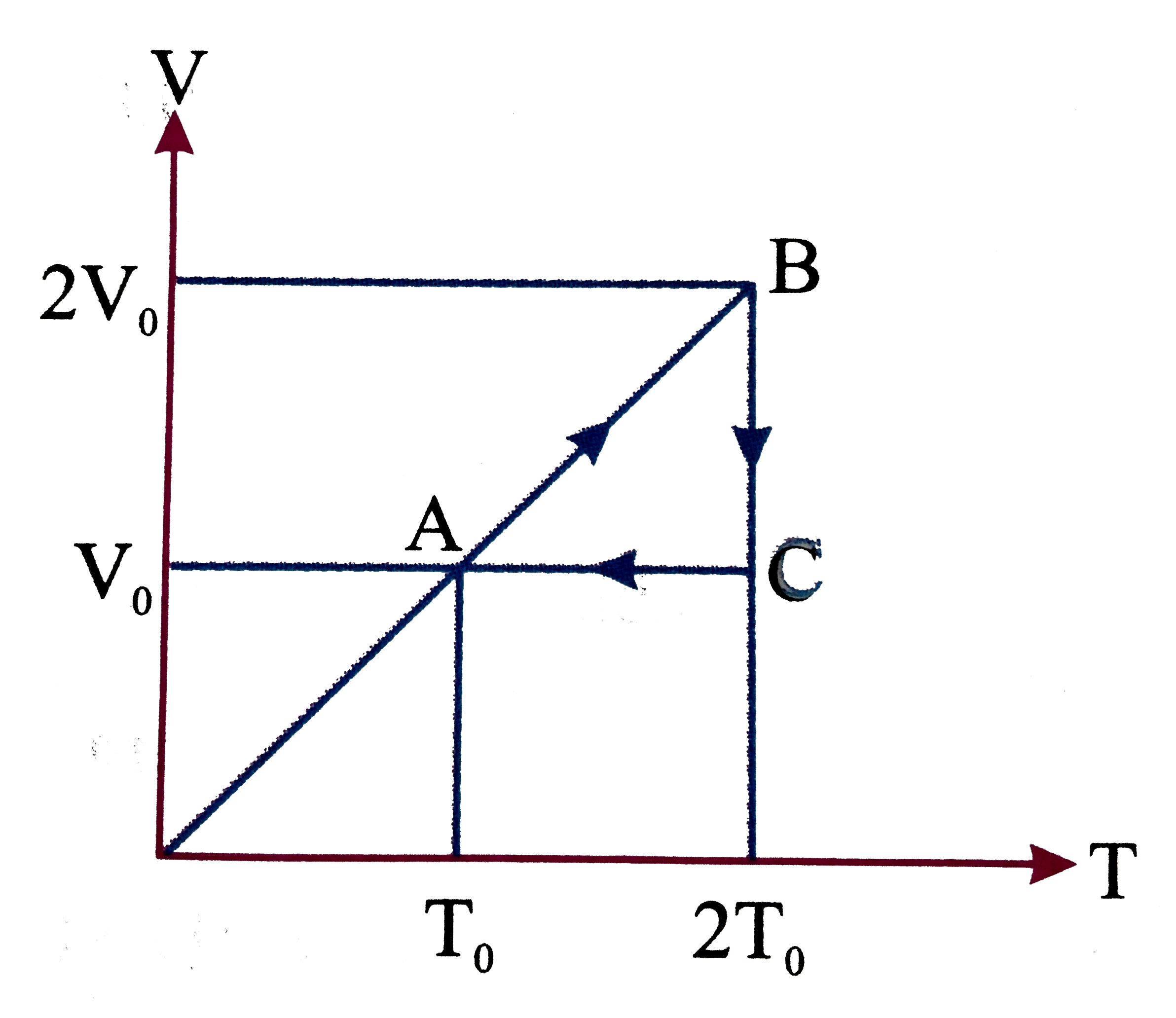
Graph of pressure versus volume of the cyclic process ABCA is
View SolutionAn ideal monoatomic gas undergoes a cyclic process ABCA as shown in the figure. The ratio of heat absorbed during AB to the work done on the gas during BC id
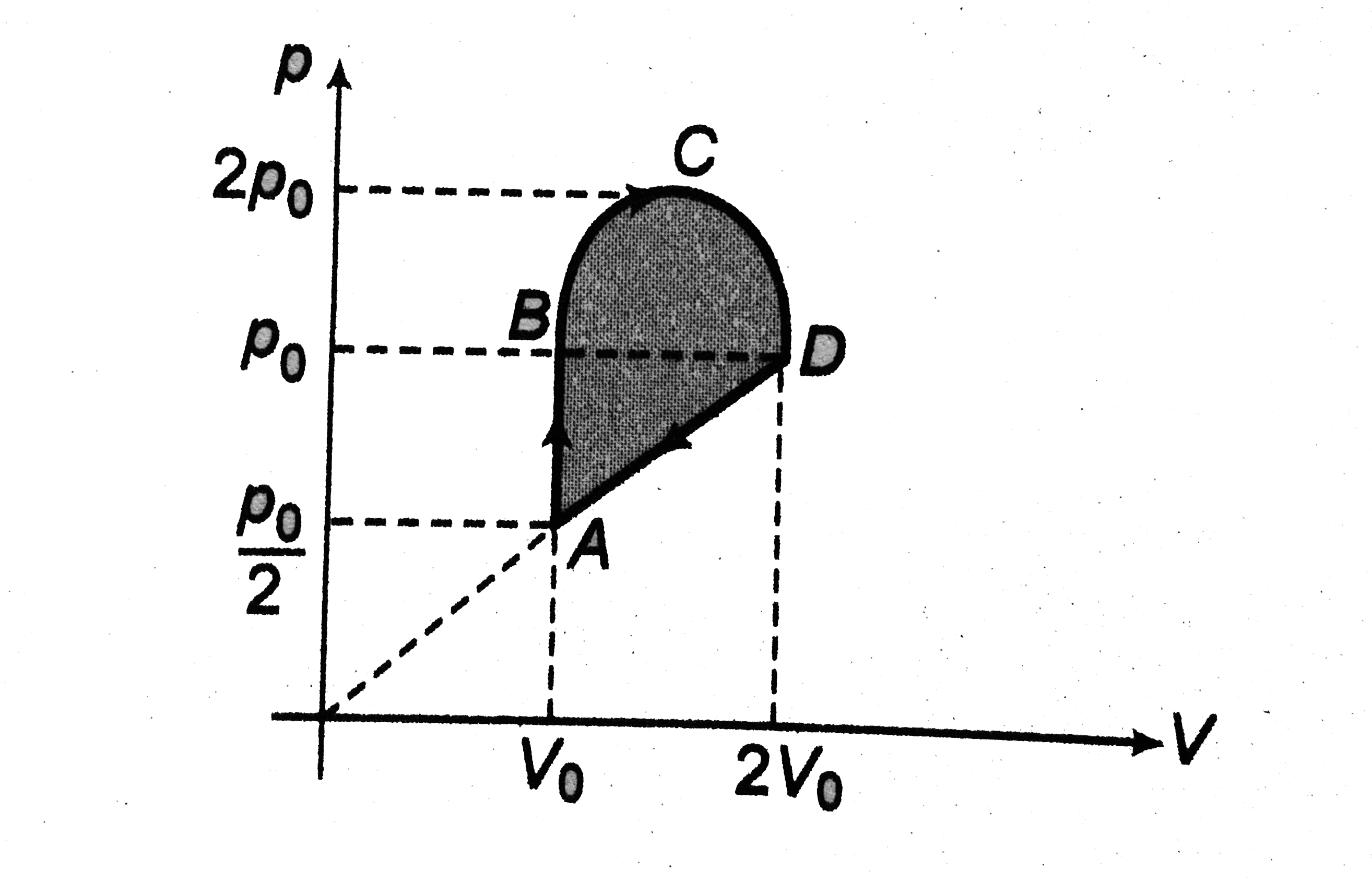
View SolutionTwo moles of a monatomic ideal gas undergo a cyclic process ABCDA as shown in figure. BCD is a semicircle. Find the efficiency of the cycle.
View Solution
