p-T diagram of one mole of an ideal monatomic gas is shown. Processes AB and CD are adiabatic. Work done in the complete cycle is

(a) 2.5RT
(b) −2RT
(c) 1.5RT
(d) −3.5RT
The correct Answer is:A
Qnet=Wnet
QAB=QCD=0
QBC=nCpΔT=(1)(52R)(4T−T)
=7.5RT
QDA=nCpΔT
=(1)(52R)(3T−5T)
=−5RT
∴ Wnet=Qnet=7.5RT−5RT
=2.5RT
Topper's Solved these Questions
- LAWS OF THERMODYNAMICSBook:DC PANDEY ENGLISHChapter:LAWS OF THERMODYNAMICSExercise:Level 2 More Than One Correct
Explore 6 Videos
- LAWS OF THERMODYNAMICSBook:DC PANDEY ENGLISHChapter:LAWS OF THERMODYNAMICSExercise:Level 2 Passage I
Explore 2 Videos
- LAWS OF THERMODYNAMICSBook:DC PANDEY ENGLISHChapter:LAWS OF THERMODYNAMICSExercise:Level 1 Subjective
Explore 24 Videos
- LAWS OF MOTIONBook:DC PANDEY ENGLISHChapter:LAWS OF MOTIONExercise:Medical entrances gallery
Explore 39 Videos
- MAGNETIC EFFECT OF CURRENT AND MAGNETISMBook:DC PANDEY ENGLISHChapter:MAGNETIC EFFECT OF CURRENT AND MAGNETISMExercise:Integer type Questions
Explore 10 Videos
Similar Questions
Knowledge Check
The cycle is shown in figure is made of one mole of perfect gas in a cylinder with a piston . The processes A to B and C to D are isochoric whereas process B to C and D to A are adiabatic, the work done in one cycle is (VA=VB=V,VC=VD=2Vand γ=5/3)
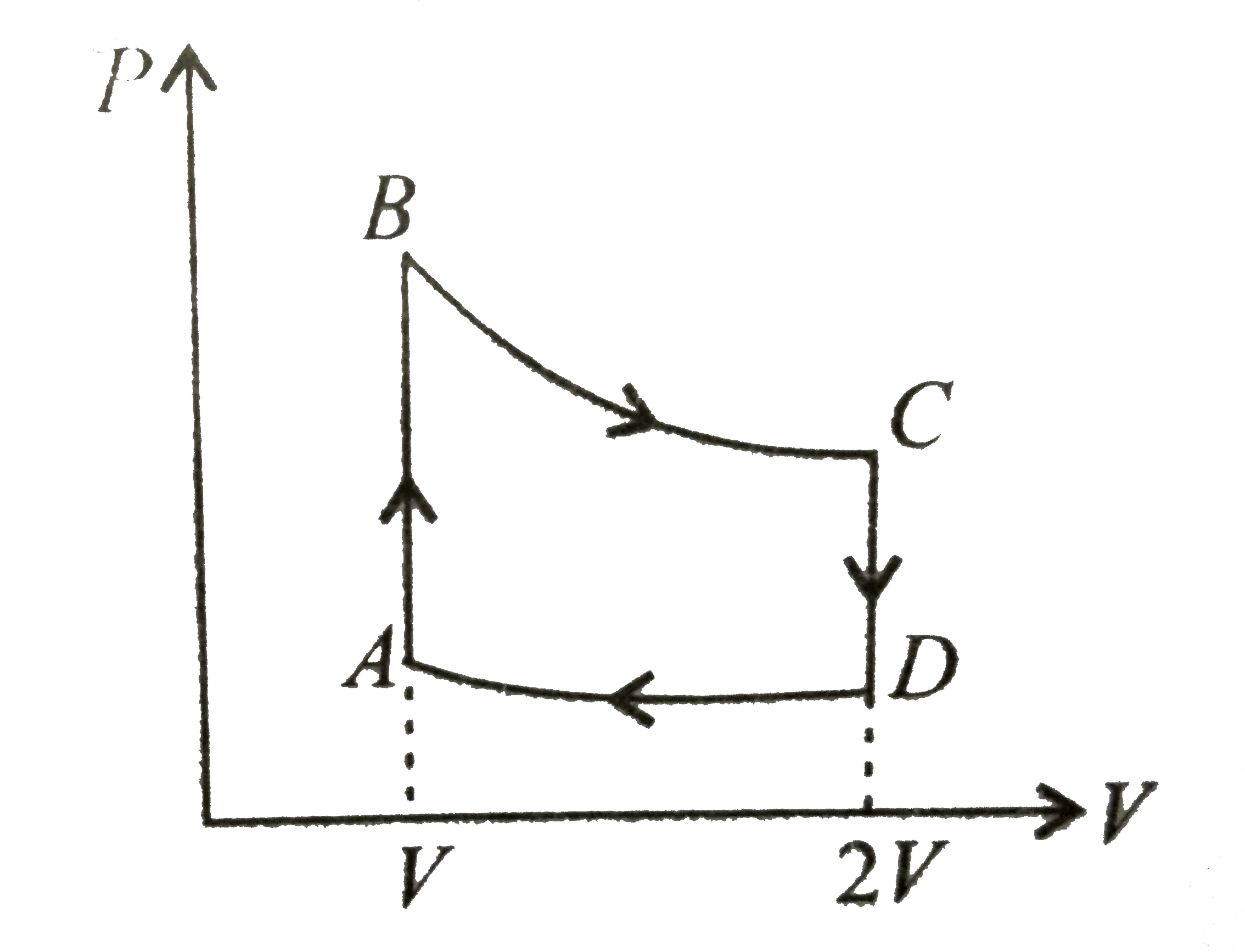 A[1−432](PB−PA)VB32[1−323](PB−PA)VC32[1−2−23](PB−PA)VD52[1−2−23](PB−PA)V
A[1−432](PB−PA)VB32[1−323](PB−PA)VC32[1−2−23](PB−PA)VD52[1−2−23](PB−PA)V
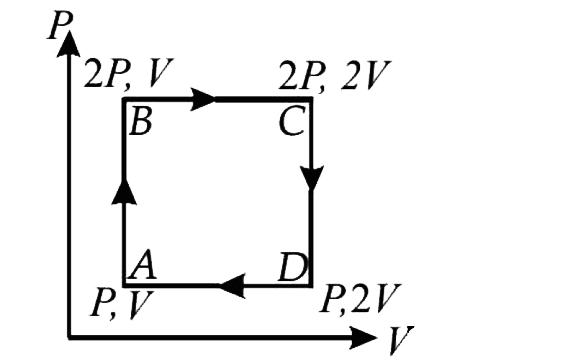
An ideal monoatomic gas is taken round the cycle ABCDA as shown in the P-V diagram. The work done during the cycle is
View SolutionOne mole of an ideal mono-atomic gas is taken round cyclic process ABCD as shown in figure below. Calculate work done by the gas.
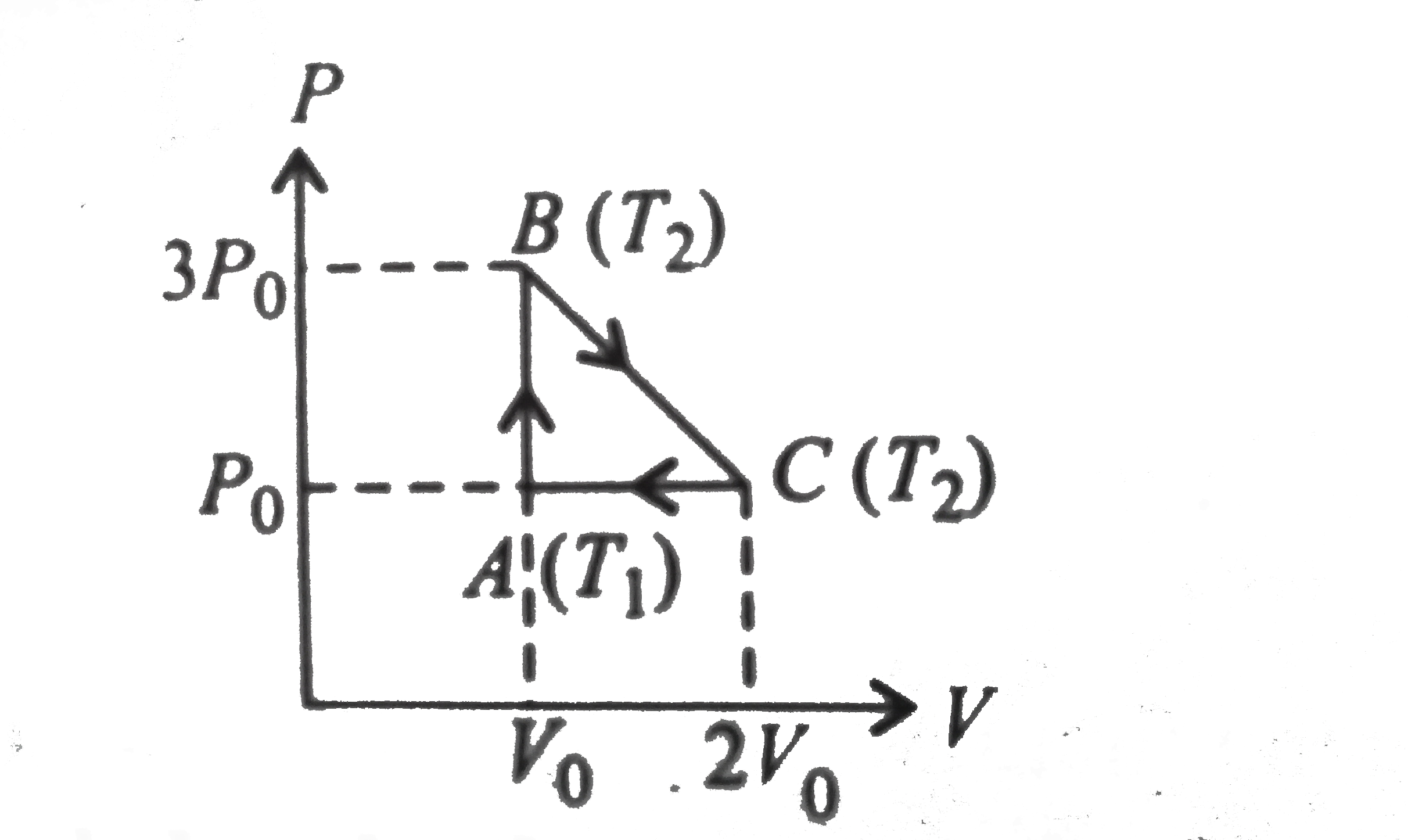
View SolutionThe work done by the system in a cyclic process involving one mole of an ideal monoatomic gas is −50kJ/cyc≤. The heat absorbed by the system per cycle is
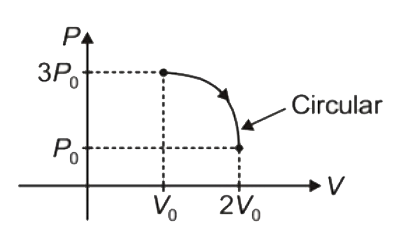
View SolutionOne mole of an ideal diatomic gas is taken through a process whose P−V diagram is shown in the figure. The work done by the gas is
View SolutionOne mole of an ideal mono-atomic gas is taken round cyclic process ABC as shown in figure below. Calculate work done
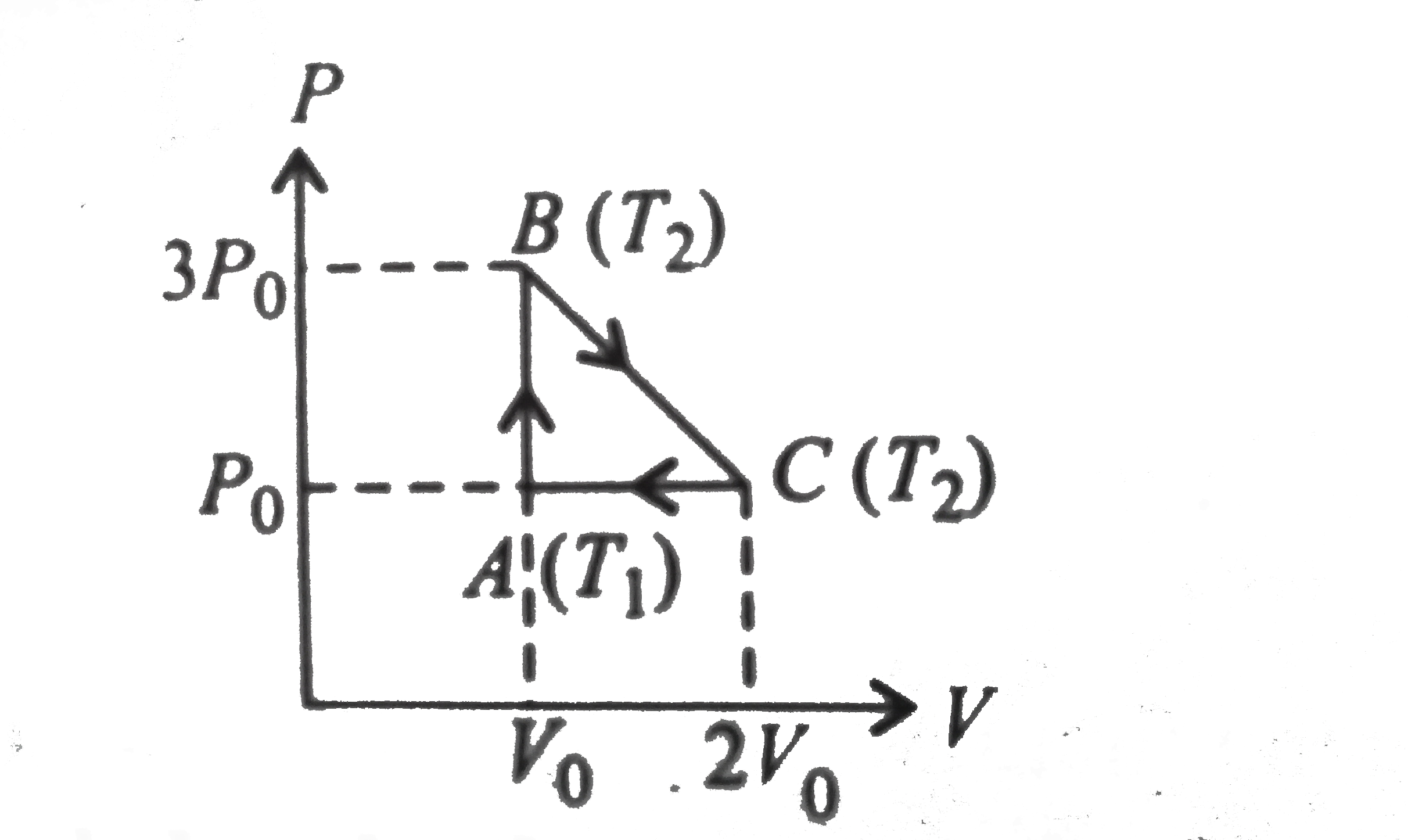
View SolutionOne mole of an ideal mono-atomic gas is taken round cyclic process ABC as shown in figure below. Calculate work done
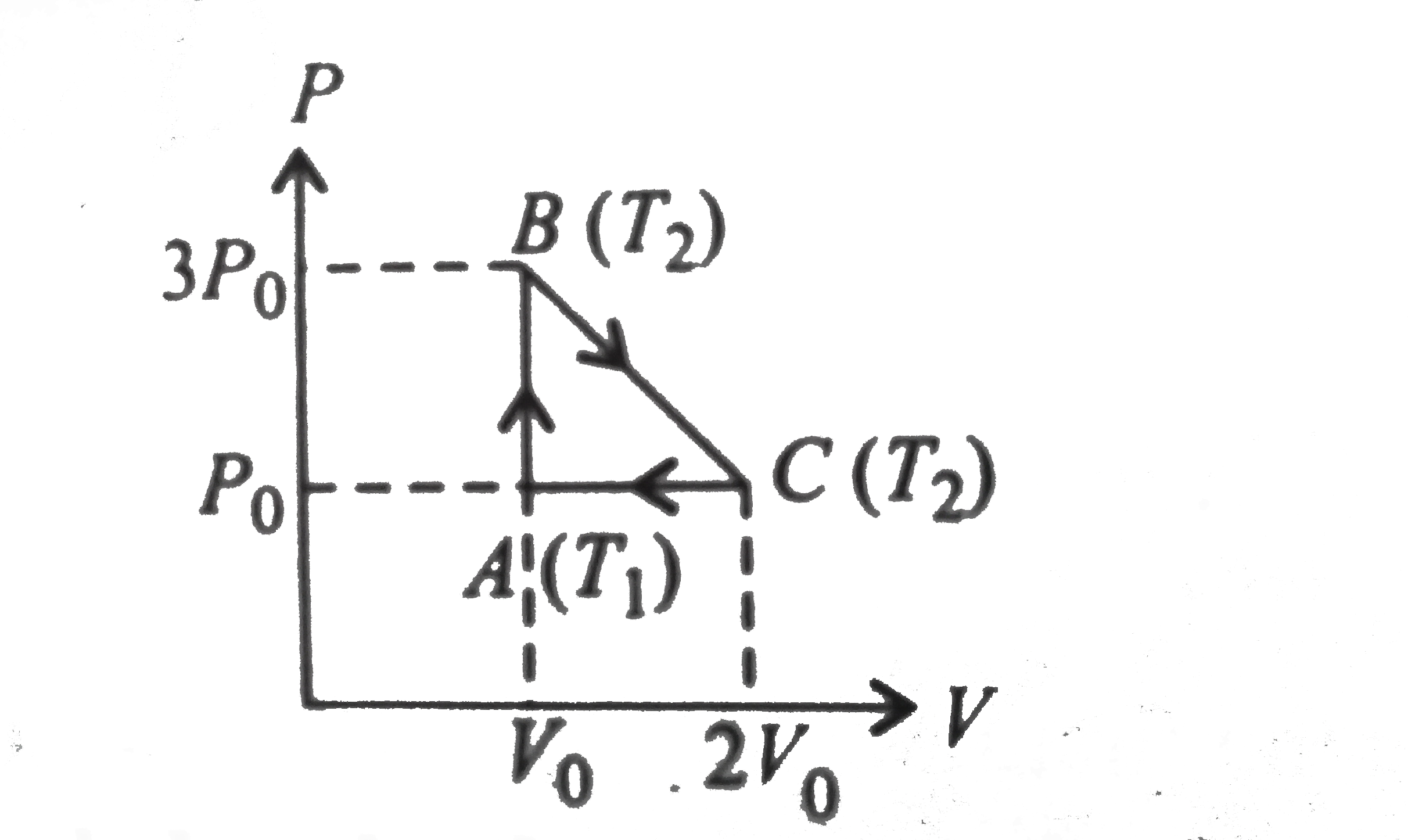
View Solutiongraph of an ideal gas is as shown in figure.
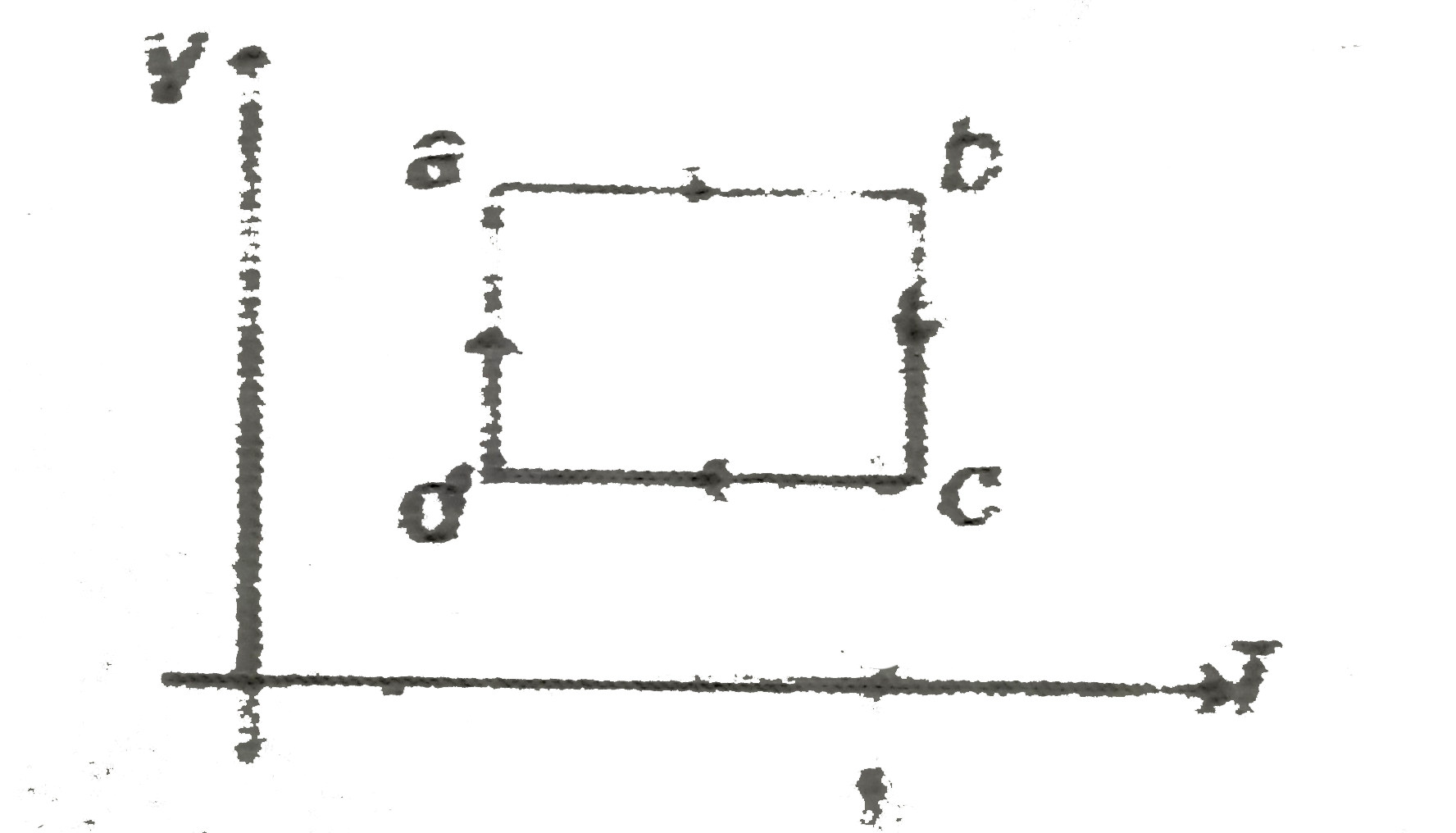
Work done by the gas in complete cyclic process about is
View Solution

