The ratio between acceleration of the electron in singlely ionized helium atom and doublely ionized lithium atom (both in ground state) is (x/27). Find value of x.
49
278
827
94
The correct Answer is:C
a=v2rora∝(Z)2(1/Z)
a∝Z3
For singly ionised helium atom, Z=2
and doubly ionised lithium atom Z=3
⇒ aHeaLi=(2)3(3)3=827
Topper's Solved these Questions
- ATOMSBook:DC PANDEY ENGLISHChapter:ATOMSExercise:ASSERTION AND REASON
Explore 10 Videos
- ATOMSBook:DC PANDEY ENGLISHChapter:ATOMSExercise:MATCH THE COLUMNS
Explore 4 Videos
- ATOMSBook:DC PANDEY ENGLISHChapter:ATOMSExercise:Check point 12.3
Explore 15 Videos
- ALTERNATING CURRENTBook:DC PANDEY ENGLISHChapter:ALTERNATING CURRENTExercise:JEE MAIN
Explore 62 Videos
- CAPACITORSBook:DC PANDEY ENGLISHChapter:CAPACITORSExercise:OBJECTIVE_TYPE
Explore 1 Video
Similar Questions
Assertion: Angular momentum of single electron in any orbit or hydrogen type atom is independent of the atomic number of the element.
Reason: In ground state angular momentum is minimum
View SolutionIn Column I physical quantities corresponding to hydrogen and hydrogen like atom are given. In column II, powers of principal quantum number n are givem on which thosee physical quantities depend. Match the two columns.
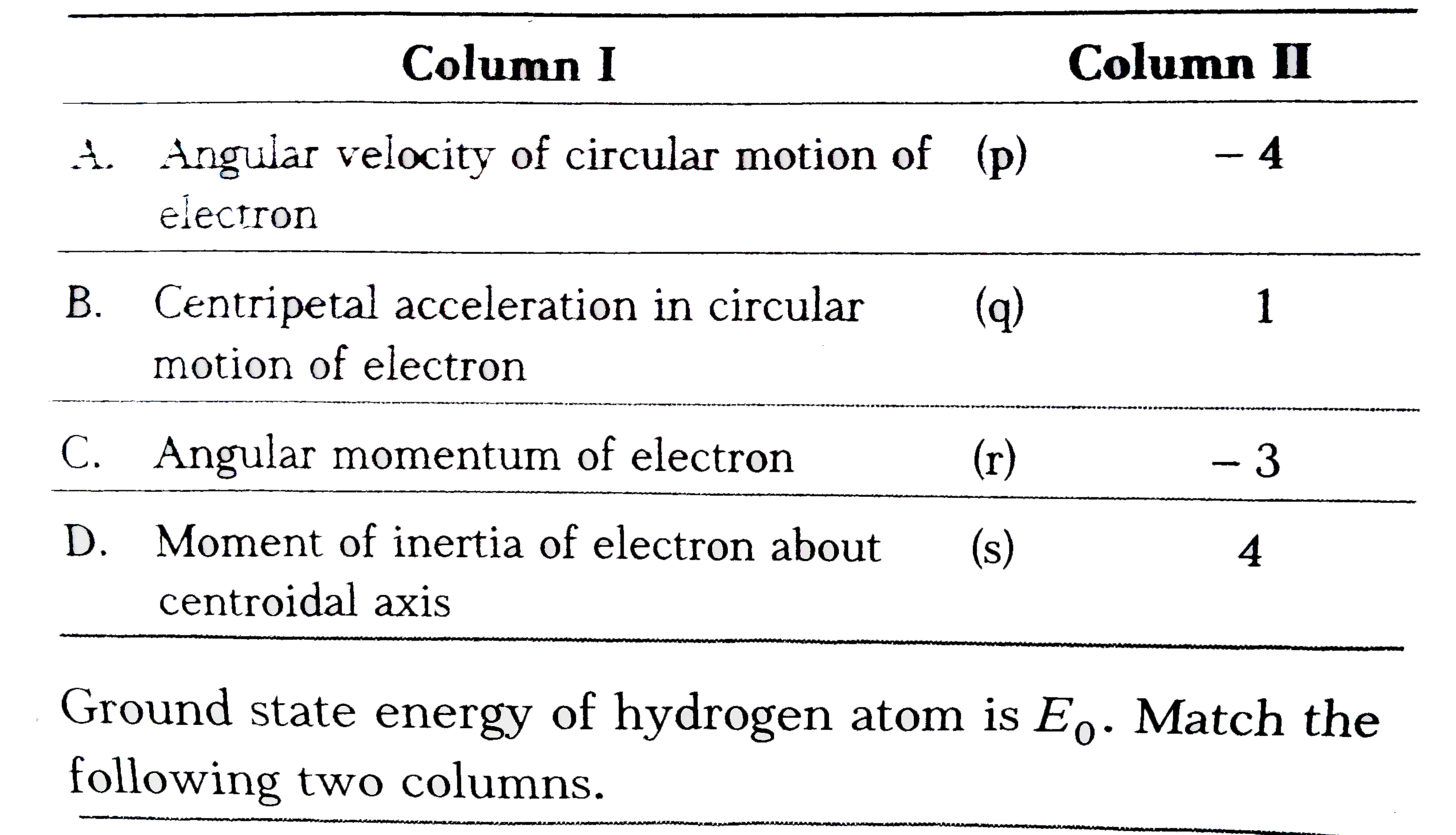
View SolutionX-rays were discovered by
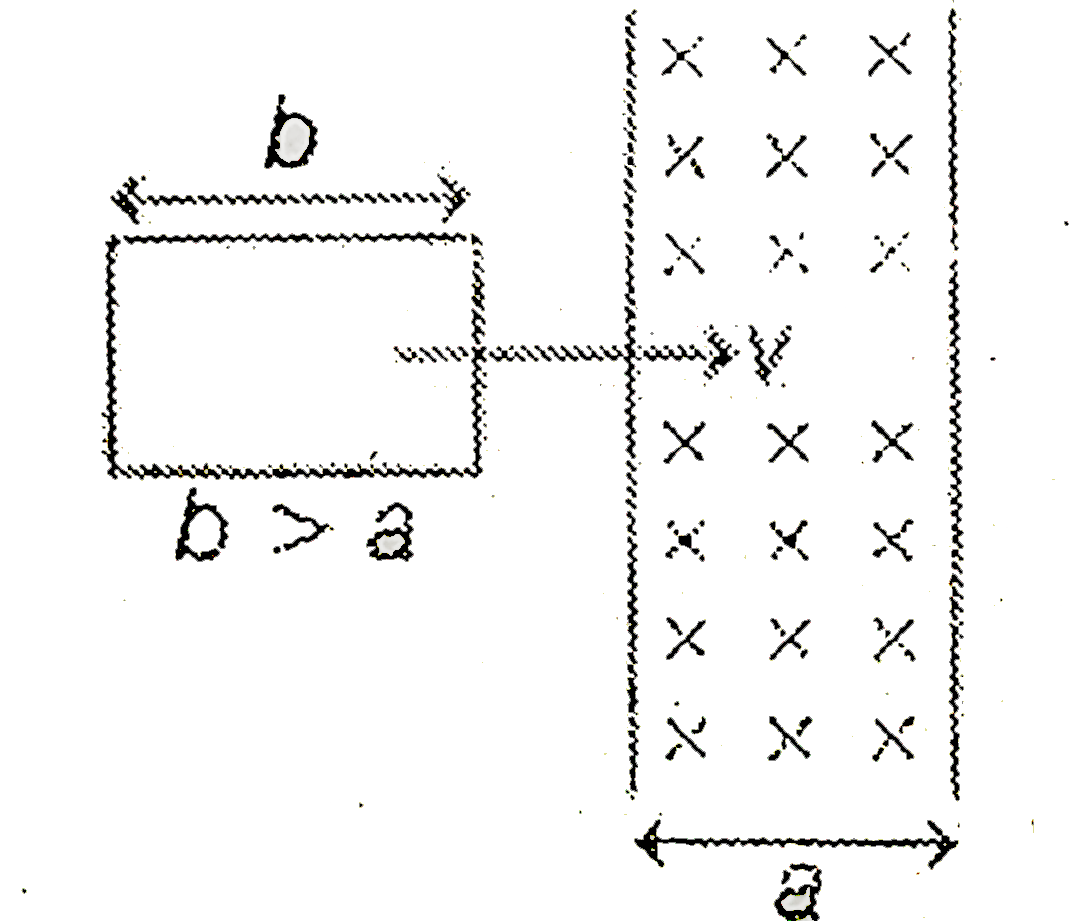
View SolutionIn the given arrangement, the loop is moved with consisant velocity in uniform magnetic field B in a restricted a region of width a. The time for which the emf is induced in the cirucit is
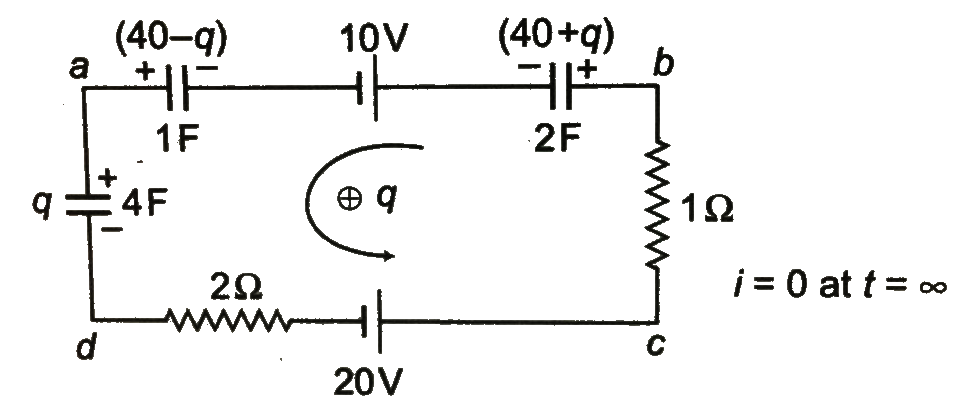
View SolutionIn the circuit shown as potential difference of 60V is appliled across AB. The potential difference between the points M and N is
View SolutionThe longest wavelength that the singly ionized helium atom in its ground state will obsorb is
View SolutionThe ratio between total acceleration of the electron in singly ionized helium atom and hydrogen atom (both in ground state) is
View SolutionThe ratio between total acceleration of the electron in singly ionized helium atom and hydrogen atom (both in ground state) is
View SolutionThe ionization potential for the electron in the ground state of the hydrogen atom is 13.6 eV "atom"^(-1). What would be the ionization potential for the electron in the first excited state of Li^(+) ?
View SolutionThe ratio between acceleration of the electron in singley ionized helium atom and duby ionized lithium aomt (both in ground state) is (x/27). Find value of n.
View SolutionThe ratio between acceleration of the electron in singly ionised helium atom and doubly ionised lithium atom (both in ground state ) is
View SolutionThe ratio of the third ionization energy of lithium and ionization energy of H atom is of H-atom and that of Li+2 ion is:
View SolutionThe acceleration of an electron in an ionized helium atom and the ratio of the acceleration of an electron in a hydrogen atom is
View Solutionकिसी एकल आयनित हीलियम परमाणु का आयनन विभव निम्न के तुल्य है:
View SolutionThe ratio between total acceleration of the electron in singly ionized helium atom and hydrogen atom (both in ground state) is
View SolutionThe ratio between acceleration of the electron in singlely ionized helium atom and doublely ionized lithium atom (both in ground state) is (x/27). Find value of x.
View Solution
