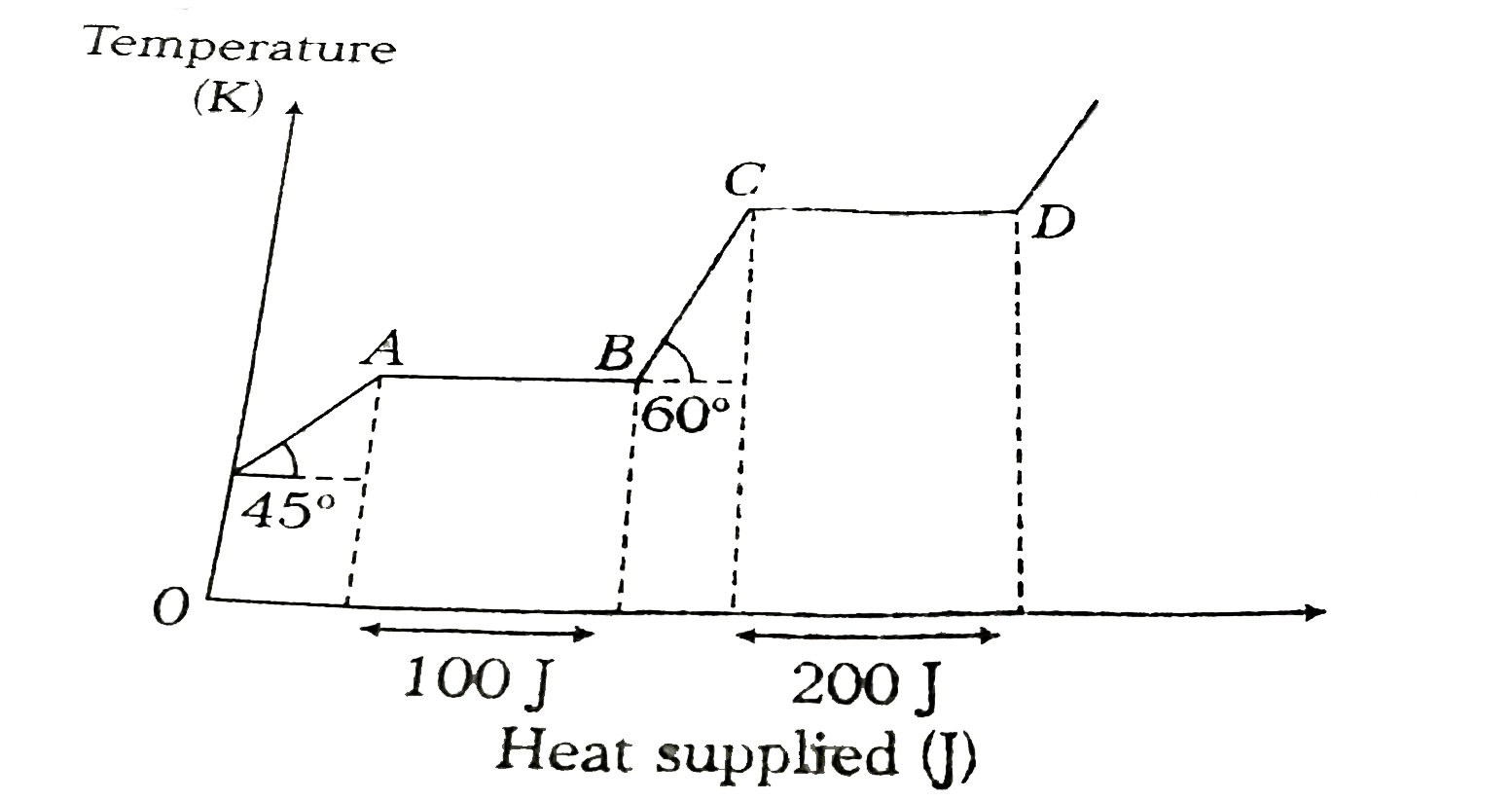
The temperature change versus heat supplied curve is given for 1 kg of a solid block. Then, which of the following statement is/are correct ?
A
Specific heat capacity of the solid is 2Jkg−1K−1
B
Specific heat capacity of liquid phase is √3Jkg−1K−1
C
Latent heat of vaporisation is 100Jkg−1
D
Latent heat of vaporisation is 200Jkg−1
Video Solution
Text Solution
The correct Answer is:D
Heat supplied, dQ = ms.dT
m = 1 kg
∴ s=dQdt = Slope of Q-T graph
Ssolid = tan45∘=1Jkg−1Ki−1
Sliquid = tan30∘ = 1√3J kg−1K−1
Latent heat of vaporisation = heat required to melt complete 1 kg = 200 J kg−1
|
Similar Questions
Knowledge Check
Which of the following statement is correct for s-block elements:
ABe has smallest atomic size in IIA groupBLi is most metallicCMg impart red colour to the flameDCs is most reducing in waterWhich of the following statements are correct about solid catalyst?
ASame reactants may give different product by using different catalystsBCatalyst does not change ΔH of reactonCCatalyst is required in large quantities to catalyse reactionsDCatalytic activity of a solid catalyst does not depend upon the strength of chemisorptionWhich of the following statements are correct about solid catalyst?
ASame reactants may give different products by using different catalystsBCatalyst does not change ΔH of reactionCCatalyst is required in large quantities to catalyse reactionsDCatalytic activity of a solid catalyst does not depend upon the strength of chemisorption
Which of the following statement regarding defects in solids is/are correct?
View SolutionWhich of the following statements are correct about the diamagnetic solids ?
View SolutionWhich of the following statement correctly relate heat and temperature?
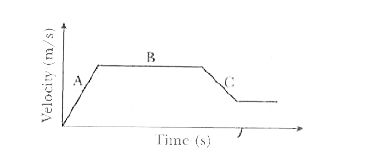
View SolutionThe given figure shows the velocity versus time curve for a car traveling along a straight line. Which of the following statements is false ?
View SolutionWhich of the following statement correctly relate heat and temperature ?
View Solution

