Two moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram
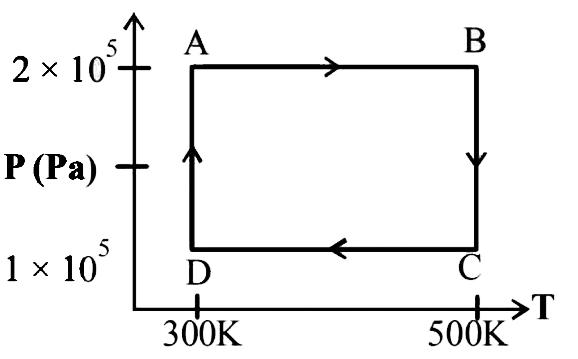
The net work done on the gas in the cycle ABCDA is :
277R
1076R
1904R
Zero
The correct Answer is:A
As WAB+WCD=0 (figure)
Wnet=WDA+WBC
=−415.9R+2R×500loge(2×1051×105)
=−415.9R+1000R×2.3030×0.3010
=−415.9R+693.02R=277.1R
Similar Questions
Two moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram The net work done on the gas in the cycle ABCDA is
View Solutionदो मोल हीलियम गैस चक्र ABCDA के लिये ली गई है जैसा P-T ग्राफ में प्रदर्शित है।
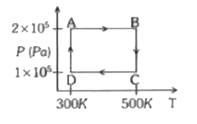
D से A तक ली गई गैस पर किया गया कार्य है
View Solutionदो मोल हीलियम गैस चक्र ABCDA के लिये ली गई है जैसा P-T ग्राफ में प्रदर्शित है।
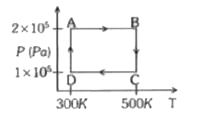
चक्र ABCDA में गैस पर किया गया कुल कार्य है
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in below in P-T diagram. Assuming the gas to be ideal , the work done on the gas in taking it from A to B is
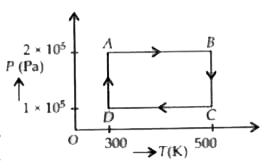
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram Assuming the gas to be ideal the work done on the gas in taking it form A to B is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram The work done on the gas in taking it from D to A is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram The net work done on the gas in the cycle ABCDA is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram
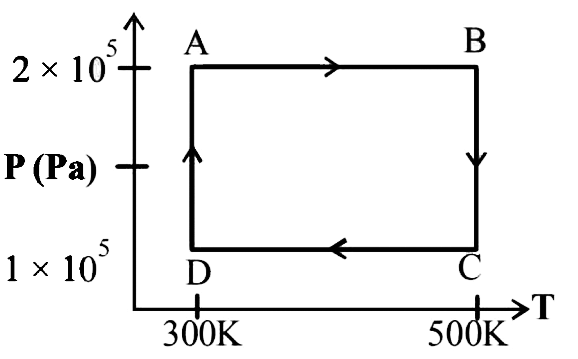
Assuming the gas to be ideal the work done on the gas in taking it form A to B is :
View Solution

