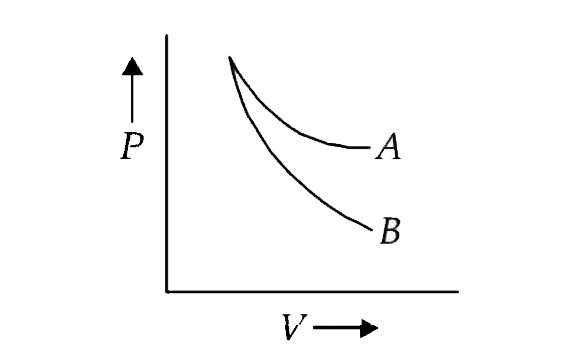
The curves A and B in the figure shown P-V graphs for an isothermal and an adiabatic process for an idea gas. The isothermal process is represented by the curve A.
Video Solution
Text Solution
The slope of P-V curve is more for adiabatic process than
for isothermal process. From the graph it is clear that slope
for B is greater than the slope for A.
|
Similar Questions
Knowledge Check
For an ideal gas, in an isothermal process
AHeat content remains constantBHeat content and temperature remain constantCTemperature remains constantDNone of the aboveIn an isothermal process for an ideal gas
AΔQ=0BΔW=0CΔU=0DΔV=0In an isothermal process for an ideal gas
AΔQ=0BΔW=0CΔU=0DΔV=0
