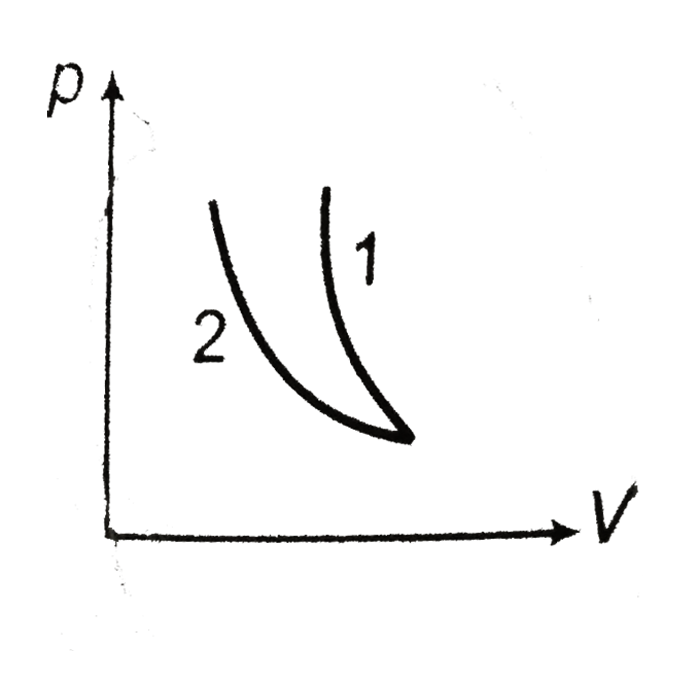
Assertion: Isothermal and adiabatic, two processes are shown on p-V diagram. Process-1 is aidabatic and process-2 is isothermal.
Reason: At a given point, slope of adiabatic process =γ×slope of isothermal process.
A
If both Assertion and Reason are true and the Reason is correct explanation of the Assertion.
B
If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
C
If Assertion is true, but the Reason is false.
D
If Assertion is false but the Reason is true.
Video Solution
Text Solution
The correct Answer is:A
|
Answer
Step by step video, text & image solution for Assertion: Isothermal and adiabatic, two processes are shown on p-V diagram. Process-1 is aidabatic and process-2 is isothermal. Reason: At a given point, slope of adiabatic process =gammaxxslope of isothermal process. by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
In isothermal process
ATemperature increasesBTemperature decreases,CTemperaturę neither increases nor decreases,DVolume remains same.An isothermal process is a
Aslow processBquick processCvery quick processDboth 1 & 2For an isothermal process
AdQ=dWBdQ=dUCdW=dUDdQ=dU+dW
