Explain the geometry of [Cu(NH3)4]2+ on the basis of hybridisation . [At. No. Cu=29]
Video Solution
Text Solution
(i) Geometry of [Cu(NH3)4]2+
Outer electronic configuration of Cu2+,
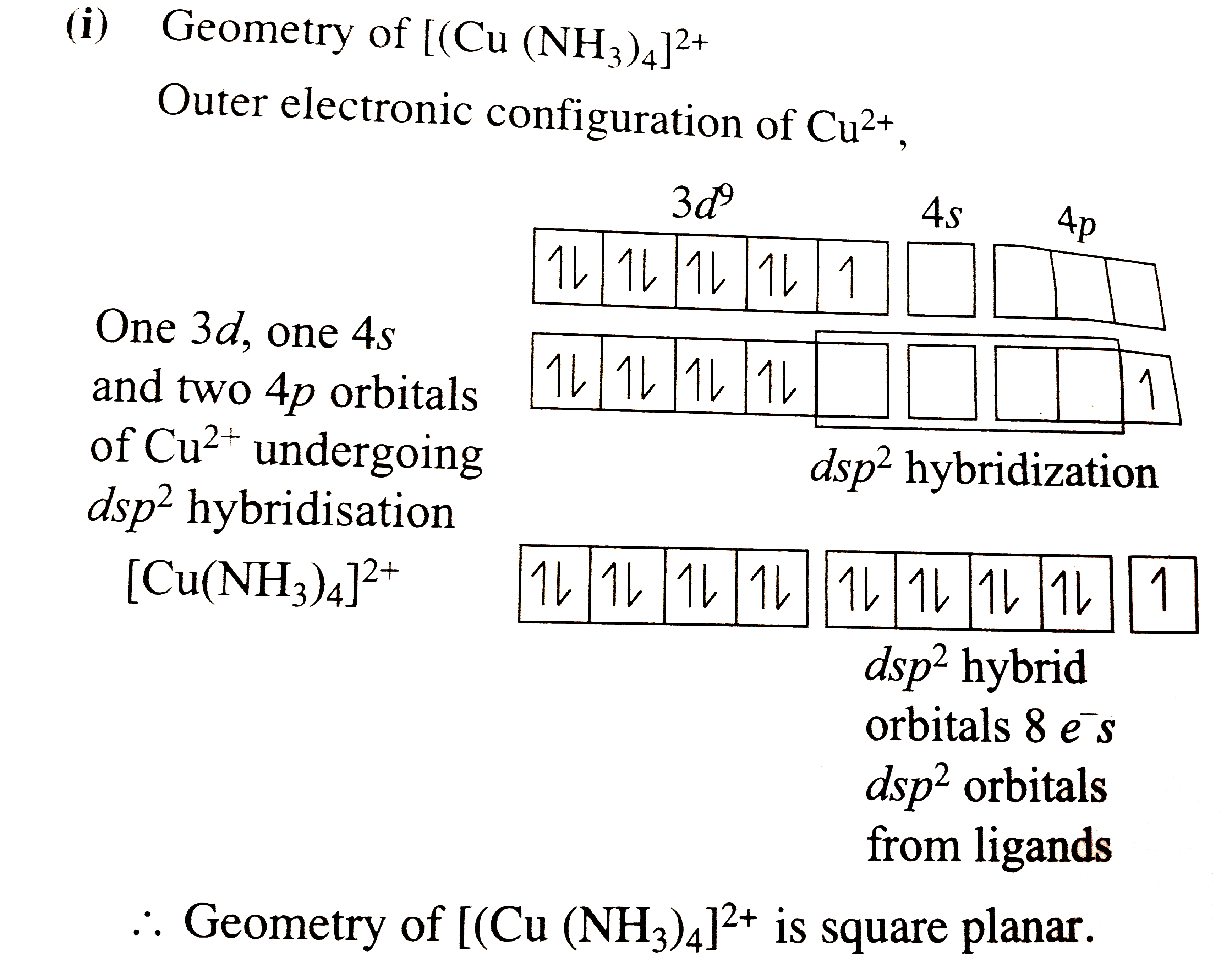
∴ Geometry of [Cu(NH3)4]2+ is square planar.
|
Similar Questions
Knowledge Check
[Cu(NH3)4]2+ shows the following hybridisation .
Adsp2Bsp3dCdsp3Dsp3The complex ion [Cu(NH3)4]2+ is
ASquare planar and diamagneticBSquare planar and paramagneticCTetrahedral and diamagneticDTetrahedral and paramagneticThe complex ion [Cu(NH3)4]2+ is
ATetrahedralBSquare planarCBothDNone of these.
