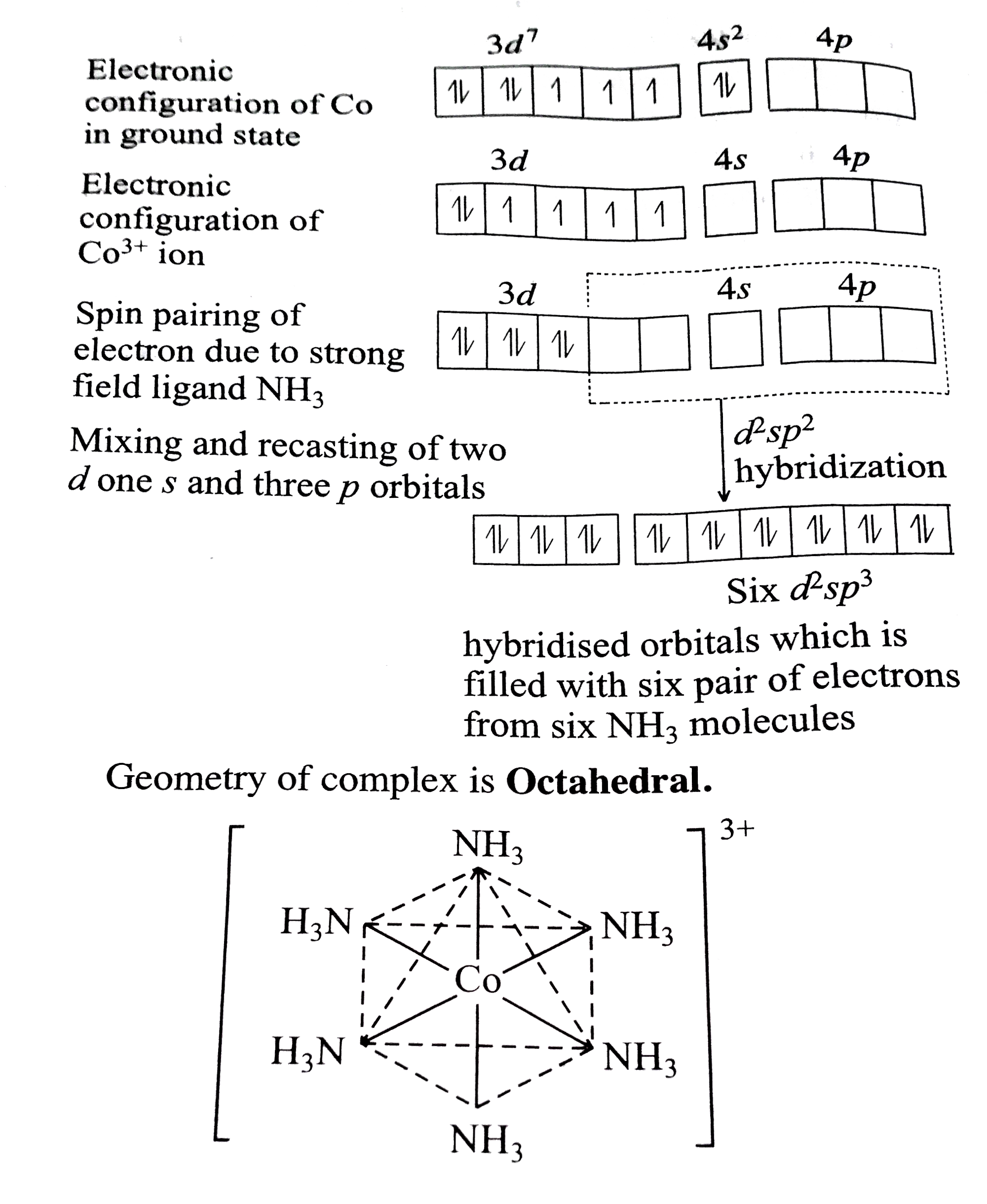
Explain the geometry of [Co(NH3)6]3+ on the basis of hybridisation. (Z of Co = 27)
Video Solution
Text Solution
|
Similar Questions
Knowledge Check
The shape of [Co(NH3)6]3+ is
ATetrahedralBTrigonal bipyramidalCOctahedralDSquare planarThe IUPAC name of [Co(NH3)6]Cl3 is
A(a) hexammine cobalt (III) chlorideB(b) hexammine cobalt (II) chlorideC(c) triammine cobalt (III) trichlorideD(d) None of theseHybridisation in [Co(NH3)6]3+ is
Asp3Bsp3dCd2sp3Dsp3d3
Discuss the shape of CO2 molecule on the basis of hybridisation.
View Solution[Cr(NH3)6]+3 complex has geometry
View Solution[Cr(CN)6][Co(NH3)6] contains
View SolutionTwo statements 'A' and 'R' are given below Select your answers to these items using the codes given below:
Assertio (A) : Among the cobalt (III) complexes [Co(NH3)6]3+ is a diamagnetic but [CoF6]3− is paramagnetic.
Reason (R) : Hybridisation of [Co(NH3)6]3+ is sp3d2 where as hybridisation of Co in [CoF6]3− is d2sp3.
View SolutionIn the compound [Co(NH3)6]Cl3 , there are _________.
View Solution