What is the correct electronic configuration of the central atom in K4[Fe(CN)6] based on crystal field theory
A
e4t22
B
t42ge2g
C
t62ge0g
D
e3t32
Video Solution
Text Solution
The correct Answer is:C
K4[Fe(CN)6]
4(+1)+x+6(−1)=0
x=+2
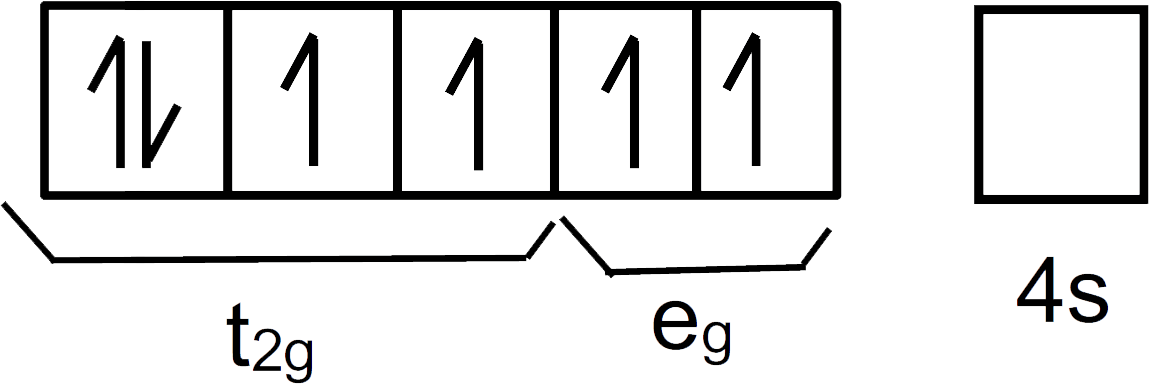
Fe2+=[Ar]3d64s0
CN− is a strong ligand due to this pairing of e occur
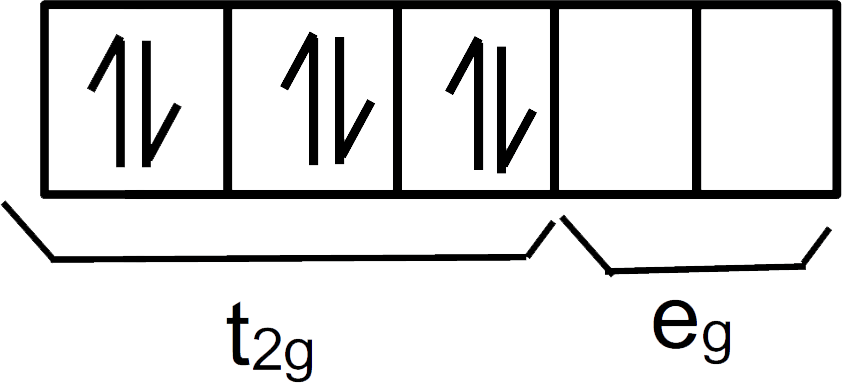
|
Topper's Solved these Questions
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:QUESTION
Explore 87 Videos
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:MCQ
Explore 91 Videos
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:MCQ
Explore 91 Videos
- METALLURGICAL OPERATIONS Book:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:METALLURGICAL OPERATIONS Exercise:MCQ
Explore 21 Videos
- NEET 2018Book:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEET 2018Exercise:QUESTION
Explore 45 Videos
Similar Questions
Knowledge Check
K4[Fe(CN)6] is a
Adouble saltBcomplex compoundCneutral moleculeDnone of thes .The bonds is K4[Fe(CN)6] are
Aall ionicBall covalentCionic, covalent and coordinateDionic and covalent.K4[Fe(CN)6] is used to detect
ACu2+BAs3+CZn2+DFe3+
