Mass action rato or reaction quotient Q for a reaction can be calculate using the law of masss action
A(g)+B(g)⇔C(g)+D(g)
Q=[C][D][A][B]
The value of Q decides whether the reaction is at equilibrium or not.
At equilibrium, Q=K
For an equilibrium process, Q≠K
when Q>K, reaction will favour backward direction and when Q<K, it will favour direction.
Answer the following questions:
For the reaction:
2A+B⇔3C at 298K,Kc=40
A 4L vessel contains 2,1, and 4 mol of A, B and c, respectively. The reaction at the same temperature
Must proceed in forward direction
Must proceed in backward direction
Must be in equilibrium
Cannot be predicted
The correct Answer is:A
2A+B⇔3C
[A]=MassVolume=24
[B]=14
[C]=44=1
∴Q=[C]3[A]2[B]=(1)2(12)2(14)=16
Therefore Q<Kc hence reaction will favour forward direction.
Similar Questions
Mass action rato or reaction quotient Q for a reaction can be calculate using the law of masss action
A(g)+B(g)⇔C(g)+D(g)
Q=[C][D][A][B]
The value of Q decides whether the reaction is at equilibrium or not.
At equilibrium, Q=K
For an equilibrium process, Q≠K
when Q>K, reaction will favour backward direction and when Q<K, it will favour direction.
Answer the following questions:
In a reaction mixture containing H2,N2 and NH3 at partial pressure of 2 atm, 1 atm and 3 atm respectively, the value of Kp at 700K is 4.00×10−5atm−2. In which direction the net reaction will go?
N2(g)+3H2(g)⇔2NH3(g)
View SolutionMass action rato or reaction quotient Q for a reaction can be calculate using the law of masss action
A(g)+B(g)⇔C(g)+D(g)
Q=[C][D][A][B]
The value of Q decides whether the reaction is at equilibrium or not.
At equilibrium, Q=K
For an equilibrium process, Q≠K
when Q>K, reaction will favour backward direction and when Q<K, it will favour direction.
Answer the following questions:
The reaction quotient Q for:
O2(g)+2H2(g)⇔2H2O(g)
is given by Q=[H2O]2[O2][H2]2. The reaction will proceed in backward direction, when
View Solution
Knowledge Check
Mass action ratio or reaction quotient Q for a reaction can be calculated using the law of mass actionl,
A(g)+B(g)⇔C(g)+D(g)
Q=[C][D][A][B]
The value of Q decides whether the reaction is at equilibrium or not.
At equilibrium, Q=K
For non-equilibrium process Q≠K
When Q>K, reaction will be favoured in backward direction and when Q<K, it will be favoured in forward direction. For the reaction : 2A+B⇔3C at 298 K, Kc=49 A 3 L vessel contains 2, 1 and 3 moles of A, B and C respectively. The reaction at the same temperature :Amust proceed in forward direction.Bmust proceed in back directionCmust be predictedDcannot be predictedMass action rato or reaction quotient Q for a reaction can be calculate using the law of masss action
A(g)+B(g)⇔C(g)+D(g)
Q=[C][D][A][B]
The value of Q decides whether the reaction is at equilibrium or not.
At equilibrium, Q=K
For an equilibrium process, Q≠K
when Q>K, reaction will favour backward direction and when Q<K, it will favour direction.
Answer the following questions:
In the reaction:
NH4Cl(g)⇔NH3(g)+HCl(g)
a graph is plotted to show that the variation or the rate of forward and backward reaction against time.
Which of following is correct?
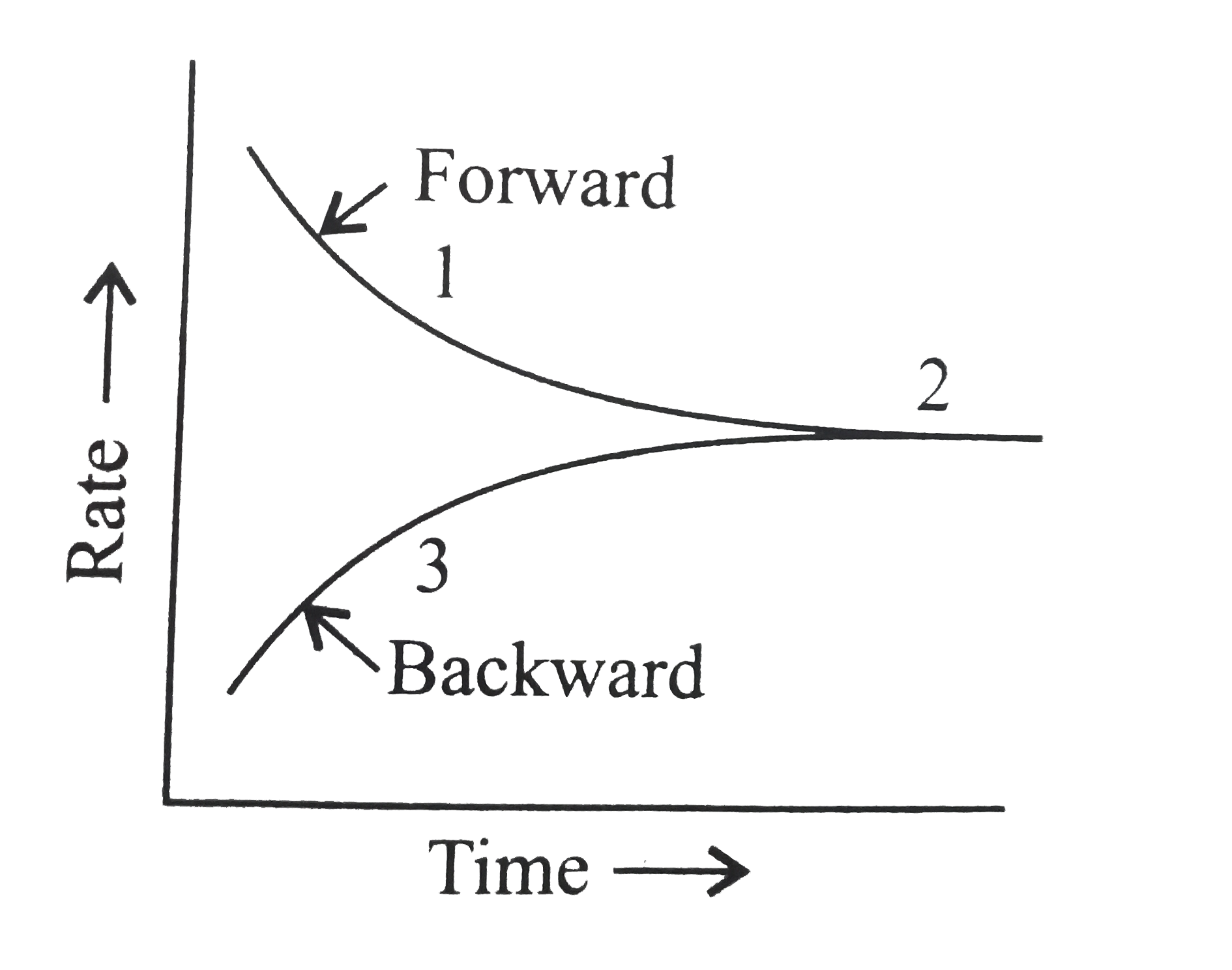 AQ>K=3,Q=K=2,Q<K=1BQ>K=2,Q=K=3,Q<K=1CQ>K=1,Q=K=2,Q<K=3DQ>K=2,Q=K=1,Q<K=3
AQ>K=3,Q=K=2,Q<K=1BQ>K=2,Q=K=3,Q<K=1CQ>K=1,Q=K=2,Q<K=3DQ>K=2,Q=K=1,Q<K=3Mass action rato or reaction quotient Q for a reaction can be calculate using the law of masss action
A(g)+B(g)⇔C(g)+D(g)
Q=[C][D][A][B]
The value of Q decides whether the reaction is at equilibrium or not.
At equilibrium, Q=K
For an equilibrium process, Q≠K
when Q>K, reaction will favour backward direction and when Q<K, it will favour direction.
Answer the following questions:
In the following reaction:
2SO2(g)+O2(g)⇔2SO3(g)
the equilibrium is not attained. The rate of forward reaction is greater than that of backward reaction. Thus, which of the following is the correct relation between Kp and Qp?AKp=QpBQp>KpCQp<KpDKp=Qp=1
Mass action ratio or reaction quotient Q for a reaction can be calculated using the law of mass actionl,
A(g)+B(g)⇔C(g)+D(g)
Q=[C][D][A][B]
The value of Q decides whether the reaction is at equilibrium or not.
At equilibrium, Q=K
For non-equilibrium process Q≠K
When Q>K, reaction will be favoured in backward direction and when Q<K, it will be favoured in forward direction.
In the following reaction :
2SO2(g)+O2(g)⇔2SO3(g)
The equilibrium is not attained. The rate of forward reaction is greater than that of backward reaction. Thus, which of the following is the correct relation between Kp and Qp?
View SolutionMass action ratio or reaction quotient Q for a reaction can be calculated using the law of mass actionl,
A(g)+B(g)⇔C(g)+D(g)
Q=[C][D][A][B]
The value of Q decides whether the reaction is at equilibrium or not.
At equilibrium, Q=K
For non-equilibrium process Q≠K
When Q>K, reaction will be favoured in backward direction and when Q<K, it will be favoured in forward direction. In a reaction mixture containing H2,N2 and NH3 at partial pressure of 2 atm, 1 atm and 3 atm respectively, the value of Kp at 725 K is 4.28×10−5 atm−2. In which direction the net reaction will go ?
N2(g)+3H2(g)⇔2NH3(g)
View SolutionThe reaction quotient (Q) at equilibrium is
View SolutionThe relation between KPandKC for the reaction A(g)+B(g)⇔C(g)+2D(g) is -
View SolutionThe Kc for given reaction will be A2(g)+2B(g)⇔C(g)+2D(s)
View Solution
