The formation of ammonia by Haber's process is favoured by ………. pressure.
High
Similar Questions
Match the following :
List-IList_II(P)Formation of ammonia by haber's process(1)increase in temperature(Q)Formation ofSO3in contact process for manufacture of H2SO4(2)Decrease in temperature(R)Formation of NO in Birk-land Eyde process for manufacture of HNO3(3)Decrease in pressure(S)Formation ofNO2(g)by following reactionNO(g)+O3⇔NO2(g)+O2(g)+200KJ(4)increase in pressure
View SolutionThe catalytic promoter used in Haber's process is
View Solution
Knowledge Check
Haber's process is used to prepare
AO2BN2CNH3DPH3The preparation of ammonia by Haber's process is an exothermic reaction. If the process follows the following temperature pressure relationship for its % yield, then for temperatures T1,T2,andT3 the correct option is
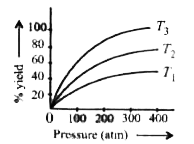 AT3>T2>T1BT1>T2>T3CT1=T2=T3Dnothing could be predicted.
AT3>T2>T1BT1>T2>T3CT1=T2=T3Dnothing could be predicted.In a catalytic reaction involving the formation of ammonia by Haber's process N2+3H2→2NH3 the rate of appearance of NH3 was measured as 2.5×10−4molL−1s−1 The rate of disappearance of H2 will be
A2.50×10−4molL−1s−1B1.25×10−4molL−1s−1C3.75×10−4molL−1s−1D5.00×10−4molL−1s−1
During synthesis of ammonia by Haber's process,
'CO' acts is
View SolutionIn which of the following reaction, the formation of product is favoured by increase in pressure?
View SolutionFormation of ammonia from H2andN2 by Haber's process using Fe is an example of
View SolutionFormation of ammonia from H2andN2 by Haber's process using Fe is an example of
View SolutionFormation of ammonia from H2andN2 by Haber's process using Fe is an example of
View Solution
