Match the following :
List-IList_II(P)Formation of ammonia by haber's process(1)increase in temperature(Q)Formation ofSO3in contact process for manufacture of H2SO4(2)Decrease in temperature(R)Formation of NO in Birk-land Eyde process for manufacture of HNO3(3)Decrease in pressure(S)Formation ofNO2(g)by following reactionNO(g)+O3⇔NO2(g)+O2(g)+200KJ(4)increase in pressure
PQRS1.42.24.13
PQRS2.42.412
PQRS4.11.233.2
PQRS2.32.312
The correct Answer is:B
Topper's Solved these Questions
- CHEMICAL EQUILIBRIUMBOOK - FIITJEECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - MATCHING TYPE QUESTIONS
3 Videos
- CHEMICAL EQUILIBRIUMBOOK - FIITJEECHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - MATRIX -MATCH TYPE QUESTIONS
3 Videos
- CHEMICAL ENERGETICS BOOK - FIITJEECHAPTER - CHEMICAL ENERGETICS EXERCISE - NUMERICAL BASED QUESTIONS
2 Videos
- CHEMICAL KINETICS AND RADIOACTIVITYBOOK - FIITJEECHAPTER - CHEMICAL KINETICS AND RADIOACTIVITYEXERCISE - Exercise
9 Videos
Similar Questions
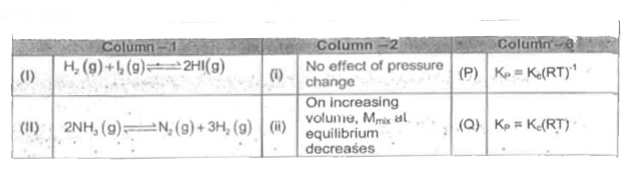

Which of the following combination is correct ?
View SolutionMatch Column-I with Column -II,
Column -IColumn-IIA2(g)+3B2(g)⇔2AB3(g)(RT)−2A2(g)+B2(g)⇔2AB(g)(RT)0A2(s)+32B2(g)⇔AB3(g)(RT)1/2AB2(g)⇔AB(g)+12B2(g)(RT)−1/2Favours forward reaction by rising pressure
View SolutionCalculate the ratio of Z in bcc of fcc?
View SolutionThe rate of formation of NO(g) in the reaction 2NOBr(h)→2NO(g)+Br2(g) was reported as 1.6×10−4mol−1sec−1. What is the rate of reaction and rate of consumption of NOBr?
View SolutionThe formation of ammonia by Haber's process is favoured by ………. pressure.
View SolutionIn the manufacture of ammonia by Haber's process, N_(2)(g) +3H_(2)(g) hArr 2NH_(3)(g)+92.3kJ , which of the following conditions is unfavourable ?
View SolutionIn ammonia formation process, due t o increase in pressure, equlibrium will shift in
View SolutionThe formation of ammonia from N2(g) and H2(g) is a reversible reaction
N2(g)+3H2(g)⇔2NH3(g)+Heat
What is the effect of increase of temperature on this equilibrium reaction
View SolutionIn contact process the formation of SO3 takes place according to the following reaction, 2SO2+O2⇔2SO3,ΔH=−45.2kcal The formation of SO3 is fovoured by
View SolutionFor the reversible reaction, N_(2)(g)+3H_(2) (g) Leftrightarrow 2NH_(3) (g)+" heat" . The equilibrium shifts in forward direction (1) (1) By increasing the concentration of NH_(3)(g) (2) By decreasing the pressure (3) By decreasing concentration of N_2(g) and H_2(g) (4) By increasing the pressure and decreasing temperature
View SolutionIn the process 2N_2O_5(g) to 4NO_2(g) + O_2(g) , at t = 10 rate of reaction w.r.t N_2O_5, NO_2 & O_2 respectively are
View SolutionIn the reaction 2N_2O_(5(g)) rarr 4NO_(2(g)) + O_2 at a certain time. The rate of formation of NO_2 is 0.08 Ms^(-1) . Find the rate of consumption of N_2O_5 , rate of formation of O_2 and the rate of the reaction.
View SolutionExplain the temperature and pressure required for manufacture of NH_3 (ammonia)by Haber's process ?
View SolutionThe formation of ammonia by Haber's process is favoured by ………. pressure.
View SolutionIn ammonia formation process, due t o increase in pressure, equlibrium will shift in
View SolutionIn the manufacture of ammonia by Haber's process,
N2(g)+3H2(g)⇔2NH3(g)+92.3kJ, which of the following conditions is unfavourable ?
View Solution

