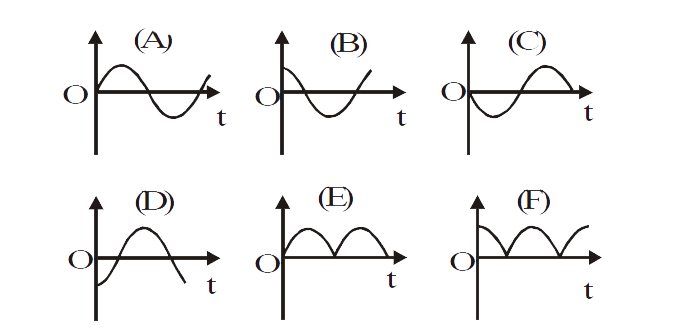
The following acid base titration graphs are given:
(I) 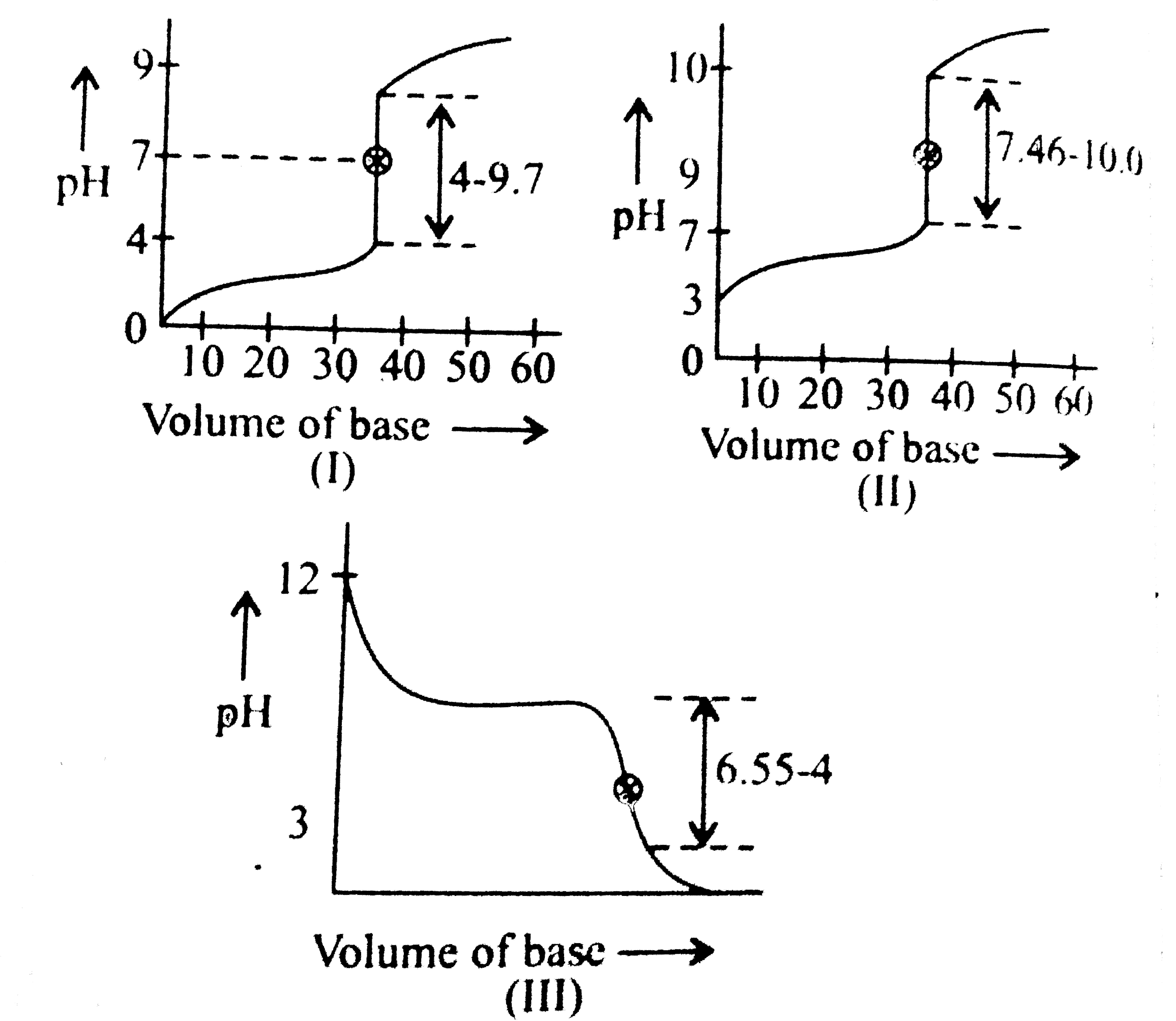
Which of the following graph represents titration of
i. NH4OH/HC1(WA/SA)
ii. HNO3/KOH(SA/SB)
iii. C6H5COOH/KOH(WA/SB)
GraphTitrationI.II.IIIi.ii.iii
GraphTitrationI.II.IIIii.iii.i
GraphTitrationI.II.IIIiii.ii.i
GraphTitrationI.II.IIIiii.i.ii
The correct Answer is:B
Step by step video, text & image solution for The following acid base titration graphs are given: (I) Which of the following graph represents titration of i. NH_(4)OH//HC1(W_(A)//S_(A)) ii. HNO_(3)//KOH(S_(A)//S_(B)) iii. C_(6)H_(5)COOH//KOH(W_(A)//S_(B)) by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- IONIC EQUILIBRIUMBOOK - CENGAGE CHEMISTRYCHAPTER - IONIC EQUILIBRIUMEXERCISE - Ex 8.5
6 Videos
- IONIC EQUILIBRIUMBOOK - CENGAGE CHEMISTRYCHAPTER - IONIC EQUILIBRIUMEXERCISE - Exercises Subjective (Weak Acid And Weak Bases)
15 Videos
- IONIC EQUILIBRIUMBOOK - CENGAGE CHEMISTRYCHAPTER - IONIC EQUILIBRIUMEXERCISE - Ex 8.3
38 Videos
- HYDROGEN, WATER AND HYDROGEN PEROXIDEBOOK - CENGAGE CHEMISTRYCHAPTER - HYDROGEN, WATER AND HYDROGEN PEROXIDEEXERCISE - Subjective Archive (Subjective)
3 Videos
- ISOMERISMBOOK - CENGAGE CHEMISTRYCHAPTER - ISOMERISMEXERCISE - Assertion-Reasoning Type
1 Video
Similar Questions
Knowledge Check
Which of the following graph represents 3s orbital
A B
B C
C D
D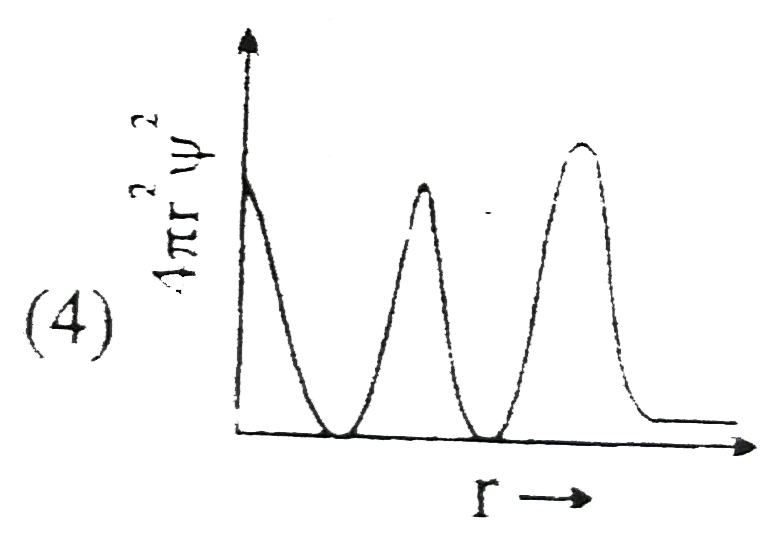
Which of the following graph represents correct graph fopr SN1 reaction:
A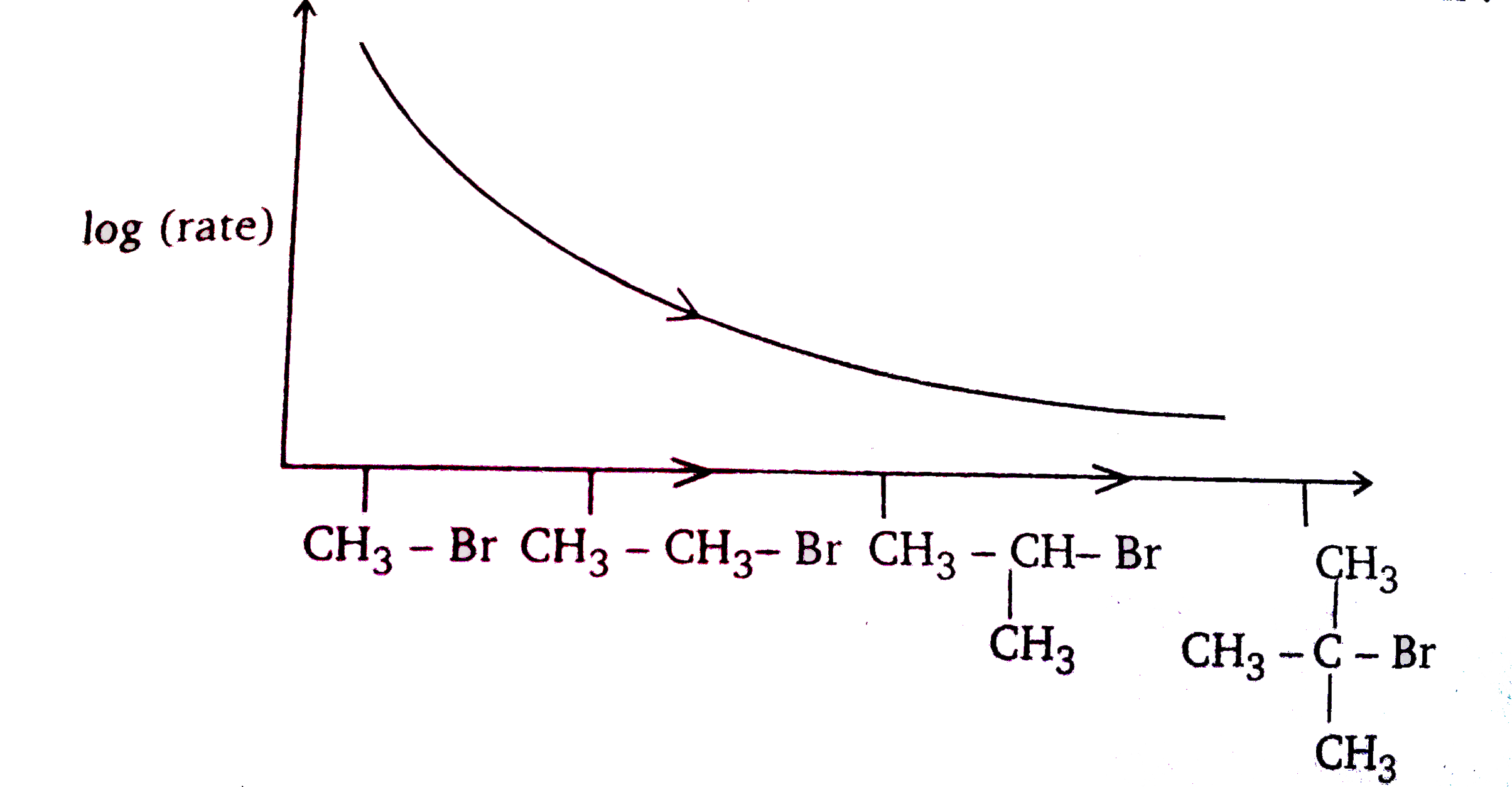 B
B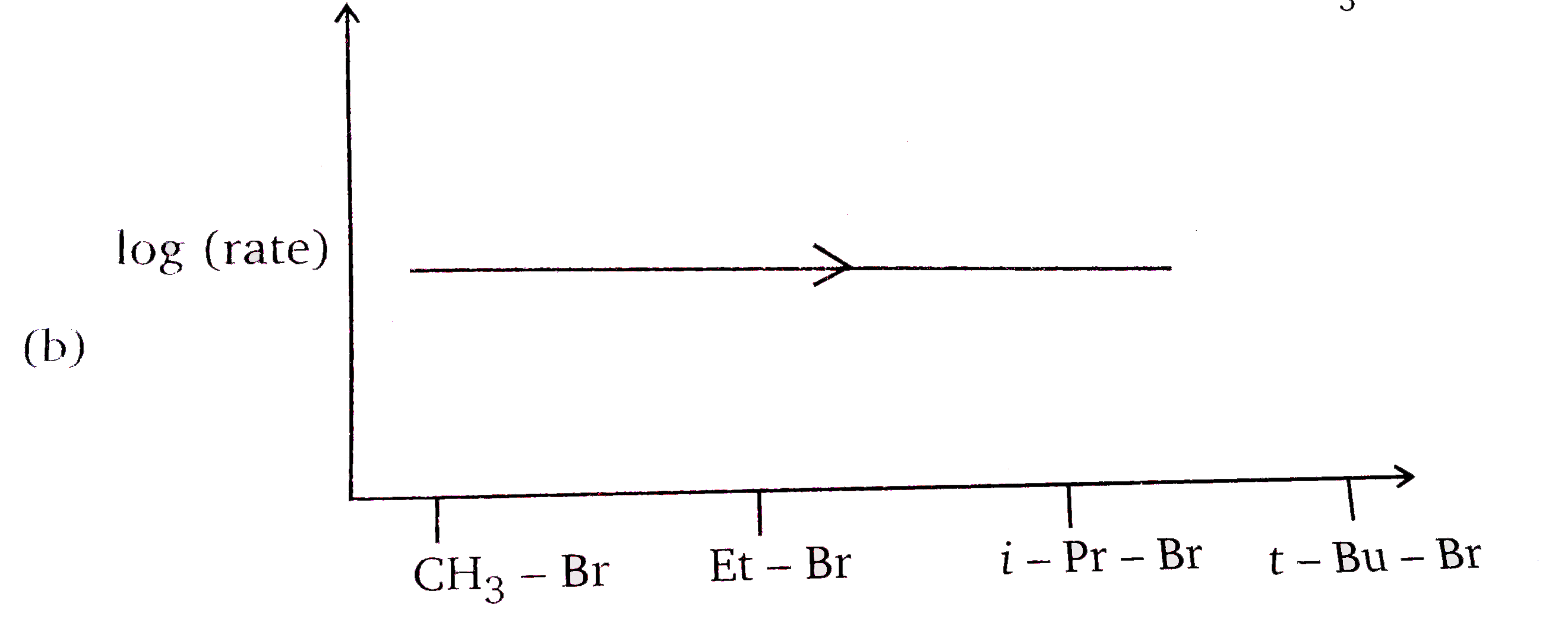 C
C D
D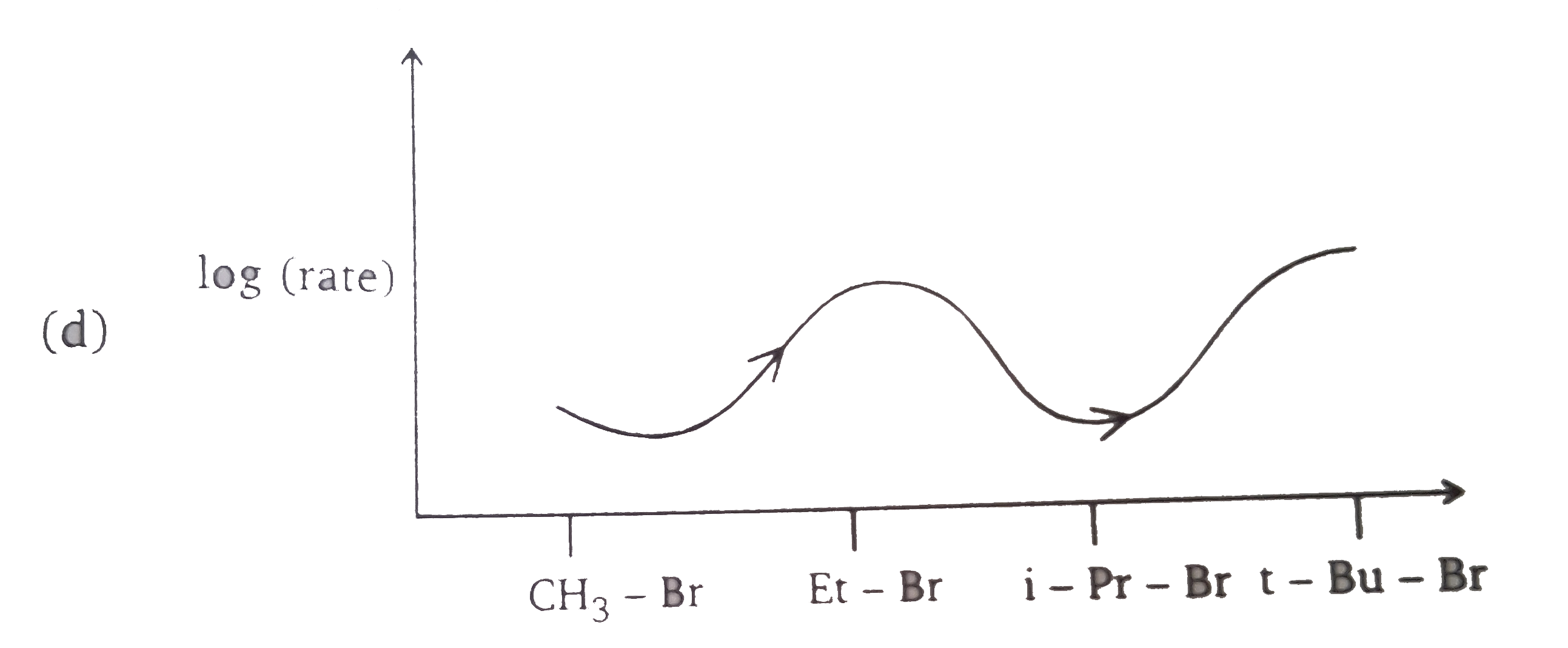
Which of the following is formed when As2S3 is warmed with NH4OHandH2O2 ?
AAs(OH)3BAsO3−4CAsO2−3D[As(NH3)6]5+
What indicators will be suitable for the following acid-base titrations:
a. HCOOH against NaOH
b. HBr against KOH
c. NH4OH with HNO3
View SolutionPredict the products of the following reactions :
(i) C6H5CH2CH3KMnO4/KOH−−−−−−−−−→H+
(ii) CH3COOHNH3/heat−−−−−−→
View SolutionWhich of the following indicators is used in the titration of Na2S2O3 against CuSO4 solution?
View SolutionThe following pH range where the indicator shows change in colour are given
i. 4−9.7 ii. 7.46−10.0 iii. 6.5−4
Which of the above pH range represent titration of
I. Strong acid/strong base (SA/SB),
II. Weak acid/strong base (WA/SB),
III. Weak base/strong acid (WB/SA)
View Solutionwhich of the following solids is not an electrical conductor ?
(a) Mg(s) (b) TiO(s) ( c) I2(s) (d) H2O(s)
View Solution