सारणी 2.4 में दिए गए मानक विभवो की सहायता से अनुमान लगाइए कि क्या इन अभिकर्मकों के बीच अभिक्रिया संभव है
(क) Fe3+(aq) तथा I−(aq)
(ख) Ag+(aq) तथा cu(s)
(ग) Fe3+(aq) तथा Cu(s)
(घ) Ag(s) तथा fe3+(aq)
(ड़) Br2(aq) तथा Fe2+(aq)
(क) संभव है -
2Fe3+(aq)+2I−(aq)→2Fe2+(aq)+I2(s)
(ख) संभव है-
Cu(s)+2Ag+(aq)→Cu2+(aq)+2Ag(s)
(ग) संभव है -
Cu(s)+2Fe3+(Aq)→Cu2+(Aq)+2Fe2+(aq)
(घ) संभव नहीं है |
(ड़) संभव है
Br2(aq)+2Fe2+→2Br−(aq)+2Fe3+(aq)
Similar Questions
Using the standard electrode potentials given in Table, predict if the reaction between the following is feasible : a. Fe^(3+)(aq) and I^(c-)(aq) b. Ag^(o+)(aq) and Cu(s) c. Fe^(3+)(aq) and Br^(c-)(aq) d. Ag(s) and Fe^(3+)(aq) e. Br_(2)(aq) and Fe^(2+)(aq) .
View Solution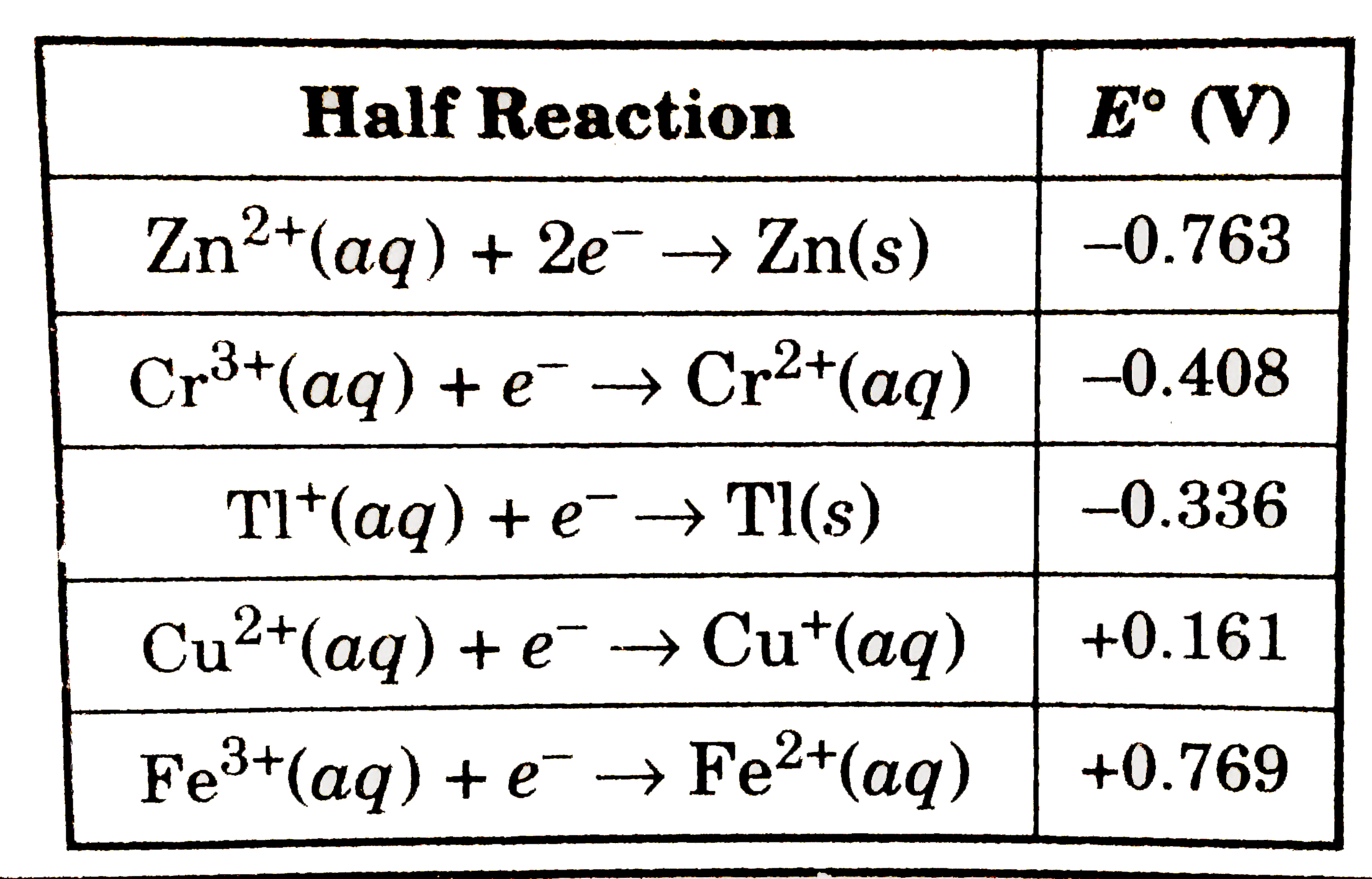
Based on the standard reduction potentials above, which reaction(s) is (are) spontaneous?
(P) Cr2+(aq)+Fe3+(aq)→Cr2+(aq)+Fe2+(aq)
(Q) Cu2+(aq)+Fe2+(aq)→Cu2+(aq)+Fe3+(aq)
View SolutionThe equilibrium constant (K) for the reaction
Fe2++(aq)Ag+(aq)→Fe3+(aq)+Ag(s) will be
Given E∘(Fe3+/Fe2+)=0.77V,
E∘(Ag+/Ag)=0.80V
View SolutionUsing the standard electrode potentials given in the table predict if the reaction between the following feasible. (a) Fe^(3+)(aq) andI^(-)(aq) (b) Ag^(+)(aq) andCu(s) (c )Fe^3+ (aq) and Cu(s) Ag(s) and Fe^(3+) (aq) (Br_(2))(aq) and Fe^(2+)(aq) (d) Ag(s) and Fe^(3+)(aq) (e) Br_(2)(aq) and fe^(2+)(aq)
View Solutionसरणी 8.1 में दिए गए मानक विभवों की सहायता से अनुमान लगाइए कि क्या इन अभिकारकों के बीच अभिक्रिया संभव है ?
(क) Fe3+ तथा I−(aq)
(ख) Ag+ तथा Cu(s)
(ग) Fe3+(aq) तथा Br−(aq)
(घ) Ag(s) तथा Fe3+(aq)
(ड़) Br2(aq) तथा Fe2+
View SolutionPredict if the reaction between the followig is feasible: (i) Fe^(3+)(aq) and I^(-)(aq) (ii) Ag^(+)(aq) and Cu (iii) Fe^(3+) and Br^(-1)(aq) (iv) Ag(s) and Fe^(3+)(aq) (v) Br_(2)(aq) and Fe^(2+)(aq) . Given standard electrode potential: E_(1//2I_(2),I^(-))^(@)=-0.541,E_(Cu^(2+),Cu)^(@)=0.34V E_(1//2Br_(2),Br^(-))^(@)=1.09V,E_(Ag^(+),Ag)^(@)=+0.80V,E_(Fe^(3+),Fe^(2+))^(@)=+0.77V
View Solutionदिए गए मानक इलेक्ट्रोड विभव का प्रयोग कर बताइए कि निम्न अभिक्रियाएं सम्भव है या नहीं-
(i) Fe3+(aq) तथा I−(aq)
(ii) Ag+(aq) तथा Cu(s)
(iii) Fe3+(aq)तथा Br−(aq)
(iv) Ag(s) तथा Fe3+(aq)
(v) Br2(aq) तथा Fe2+(aq).
View SolutionUsing the standard electrode potentials given in the predict if the reaction between the following if feasible : (a) Fe^(3+) (a) and I^(-)(aq), (b)ag^(+)(aq)and Cu(s), (c )Fe^(3+)(aq)and Cu (s) (d) Ag(s)and Fe^(3+)(aq),(e )Br_(2)(aq)and Fe^(2+)(aq)
View SolutionUsing the standard electrode potentials given in Table, predict if the reaction between the following is feasible:
a.Fe3+(aq) and Ic−(aq)
b. Ag⊕(aq) and Cu(s)
c. Fe3+(aq) and Brc−(aq)
d. Ag(s) and Fe3+(aq)
e.Br2(aq) and Fe2+(aq).
View SolutionUnder standard conditions, the following reactions occur spontaneously :
(i) Zn(s)+2Ag+(aq)→Zn2+(aq)+2Ag(s)
(ii) Fe(s)+Cu2++(aq)→Cu(s)+Fe2+(aq)
(iii) Cu(s)+2Ag+(aq)→Cu2+(aq)+2Ag(s)
(iv) Zn(s)+Cu2+(aq)→Cu(s)+Zn2+(aq)
(v) Zn(s)+Fe2+(aq)→Fe(s)+Zn2+(aq)
(vi) Fe(s)+2Ag+(aq)→Fe2+(aq)+2Ag(s)
If E∘Zn2+∣Zn,E∘Fe2+∣Fe,E∘Ag+∣AgandE∘Cu2+∣Cu have value of x, y, z and p volt respectively, then -
View Solutionनिम्नलिखित अभिक्रियाओं वाले गैल्वेनिक सेल का मानक सेल विभव परिकलित कीजिए।
2Cr(s)+3Cd+2(aq)→2Cr+3(aq)+3Cd(s)
Fe+2(aq)+Ag+(aq)→Fe+3(aq)+Ag(s)
E^0(Cd+2/Cd)= -0.4
E^0(Cr+3/Cr)= -0.74
E^0(Ag+/Ag)= 0.8
E^0(fe+3/fe+2)= 0.77
View SolutionFor the reaction, Fe^(+2) (aq) + Ag^(+) (aq) rarr Ag(s) + Fe^(+3) (aq) Predict which change will decrease the cell voltage?
View SolutionUsing the standard electron potentials given in the Table 8.1, predict if the reaction between the following is feasible : (a) Fe^(3+) (aq) and I^(-) (aq) (b) Ag^(+)(aq) and Cu(s) (c) Fe^(3+) (aq) and Cu (s) (d) Ag(s) and Fe^(3+)(aq) (e) Br_(2) (aq) and Fe^(2+) (aq)
View SolutionUsing the standard electrode potentials given is the Table 1 predict if the reaction between the following is feasible :
(a) Fe3+(aq)andI−(aq)
(b) Ag+(aq)andCu(s)
(c) Fe3+(aq)andCu(s)
(d) Ag(s)andFe3+(aq)
(e) Br2(aq)andFe2+(aq) .
View SolutionUsing the standard electrode potentials, predict if the reaction between the following is feasible :
(a) Fe3+(aq) and I−(aq), (b) Ag+(aq) and Cu(s)
(iii) Fe3+(aq) and Br− (aq), (d) Ag (s) and Fe3+ (aq)
( c) Br2(aq) and Fe2+ (aq).
Given standard electrode potentials:
E∘1/2,I2=0.541V,E∘Cu2+,Cu=0.34V
E∘1/2,Br2,Br−=1.09V,E∘Ag+/Ag=0.80V
E∘Fe3+,Fe2+=0.77V
View Solution

