Find the EMF of the concentration cell represented as given below:
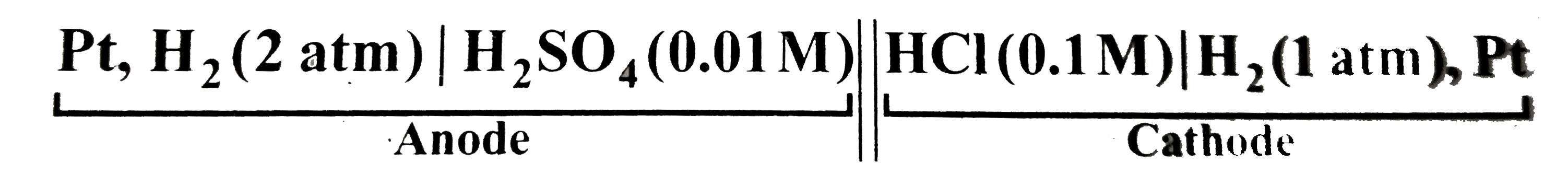
Video Solution
Text Solution
Ecell will be positively only when
[H⊕]cathode>[H⊕]anode and (pH2)anode>(pH2)cathode
So in the above case, Ecell will be positive, and cell will be spontaneous or feasible or exergonic.
[H⊕]anode=2×0.01M=2×10−2N
(n fact o r fo r H_(2)SO_(4)=2)<br>pH_(anode)=-log(2xx10^(-2))=2-0.3=1.7<br>[H^(o+)]_(cathode)=1xx0.1M" "(nfac→rforHCl=1)<br>pH_(cathode)=-log(10^(-1))=1<br>Usetherelation,<br>E_(cell)=0.059[(pH_(anode)-pH_(cathode ))+(1)/(2)log .((p_(H_(2)))_(anode))/((p_(H_(2)))_(cathode))]<br>=0.059(1.7-1+(1)/(2)log.(2atm)/(1atm))<br>=0.059(0.7+(1)/(2)log 2)<br>=0.059(0.7+(1)/(2)xx0.3)<br>=0.059(0.7+0.15)<br>=0.059xx0.85~~0.06xx0.85=0.05V
|
Similar Questions
Knowledge Check
A concentration cell is shown below
AAg(s)|AgNO3(0.01M)||AgNO3(0.001M)|Ag(s)
The EMF of the cell will b eB59.00voltsC5.90voltsD0.059voltsWhch represent a concentration cell?
APtH2|HCl||HCl|PtH2BPtH2|HCl|∣Cl2PtCZn∣∣Zn2+∣∣∣∣Cu2+∣∣CuDFe∣∣Fe+2∣∣∣∣Cu2+∣∣CuWhch represent a concentration cell?
APtH2|HCl||HCl|PtH2BPtH2|HCl|∣Cl2PtCZn∣∣Zn2+∣∣∣∣Cu2+∣∣CuDFe∣∣Fe+2∣∣∣∣Cu2+∣∣Cu
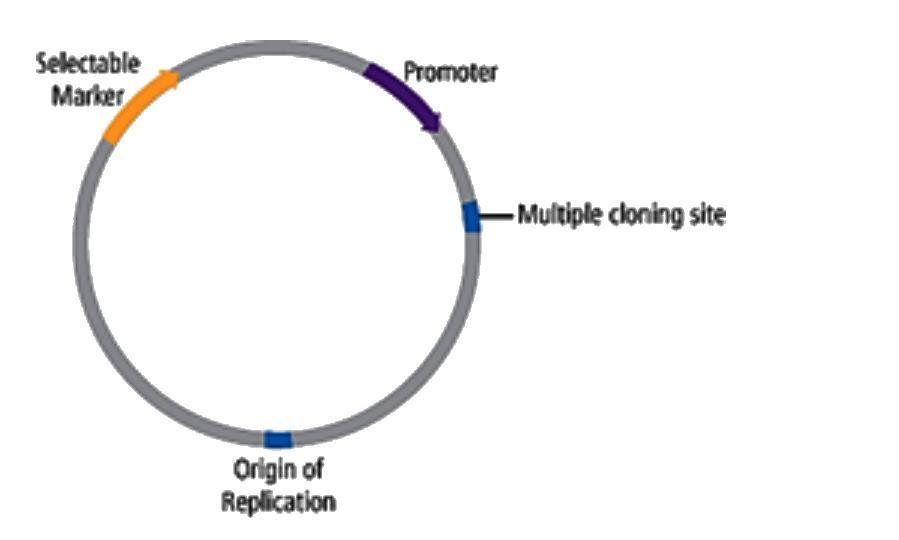
Identify the type of vector represented in the diagram given below.
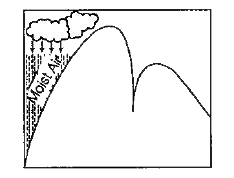
View SolutionWhich type of rainfall is represented in the diagram given below?
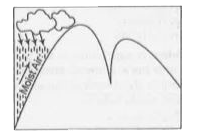
View SolutionWhich type of rainfall is represented in the diagram given below?
View SolutionThe EMF of a cell is
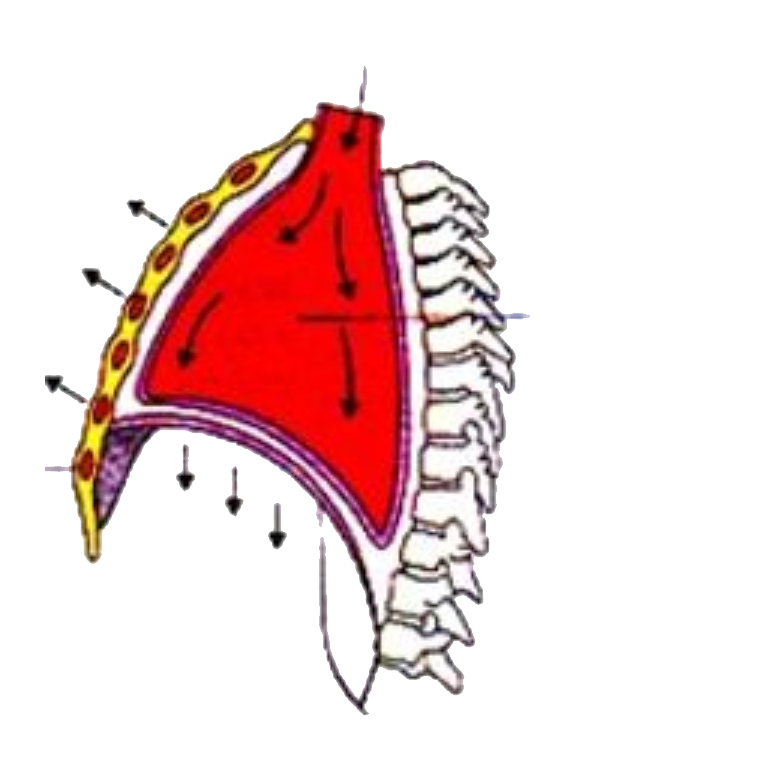
View SolutionThe diagram given below represents
View Solution