The EMF of a cell is
A
sum of two oxidation potentials
B
sum of two potentials
C
difference of two electrode potentials
D
None of the above
Video Solution
Text Solution
The correct Answer is:C
|
Answer
Step by step video, text & image solution for The EMF of a cell is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The best instrument for the accurate measurement of the e.m.f. of a cell is
APotentiometerBMetre bridgeCVoltmeterDAmmeter and voltmeterThe EMF of the cell cannot be measured accurately by a voltmeter because
Avoltmeter is very sensitiveBconcentration changes during measurementCthe voltmeter damages the cellDthe voltmeter changes the cell reactionAssertion (A: Cell constant is the EMF of a cell.
Reason (R): Cell constant is determined by using saturated KCl solution.AIf both (A) and (R) are correct, and (R) is the correct explanation of (A).BIf both (A) and (R) are correct, but (R) is not the correct explanation of (A).CIf (A) is correct, but (R) is incorrect.DIf (A) is incorrect, (R) is correct.
The e.m.f. of the cell
Ti∣∣Ti+(0.001M)∣∣∣∣Cu2+(0.01M)∣∣Cu is 0.83V the emf of this cell could b e increased by
View SolutionConsider the following cell with hydrogen electrodes at difference pressure p1 and p2.
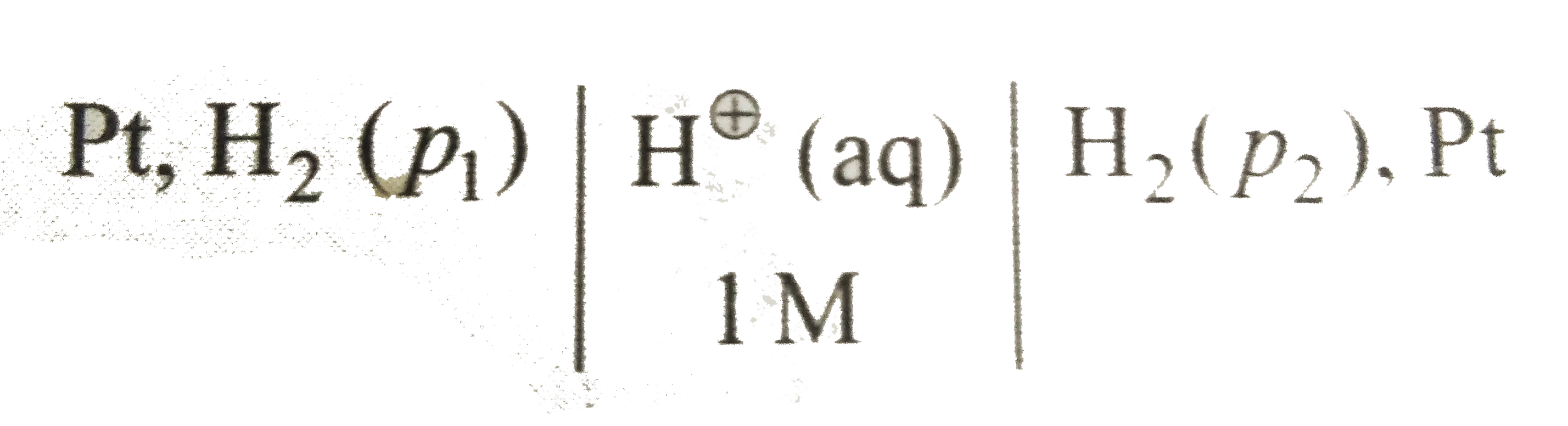
The EMF of the cell is given by
View SolutionNernst equation halps us to understand the effect of ______ on the electrode of the half-cell and emf of the voltiv cell
View SolutionDaniel cell, the EMF of the cell can b e increased b y
View SolutionDaniel cell, the EMF of the cell can b e increased b y
View Solution

