Consider the following cell with hydrogen electrodes at difference pressure p1 and p2.
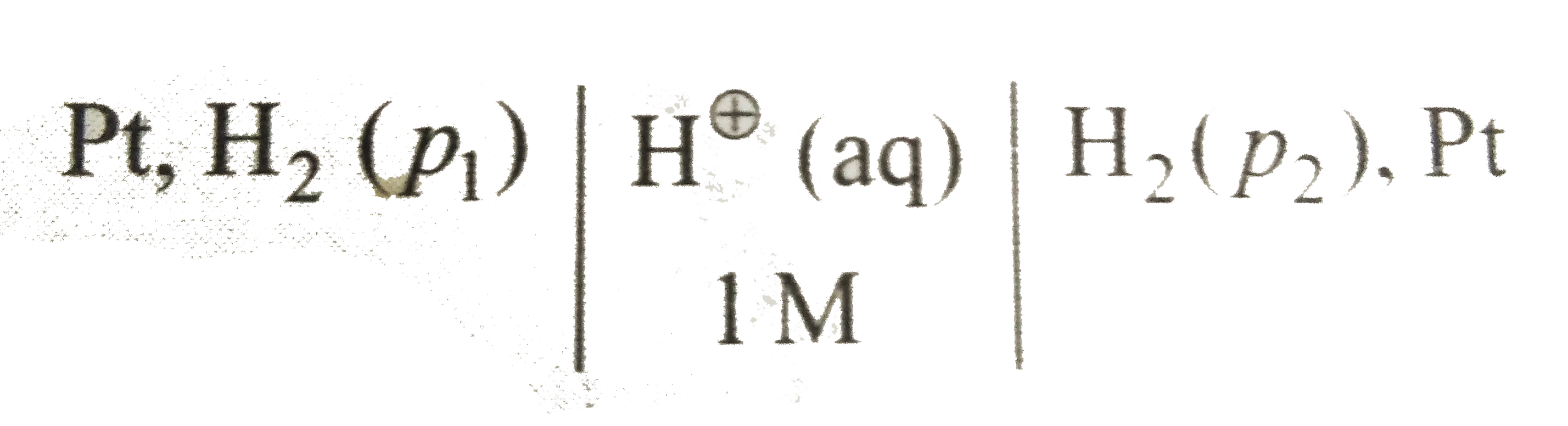
The EMF of the cell is given by
RTFln.p1p2
RT2Fln.p1p2
RTFln.p2p1
RT2Fln.p2p1
The correct Answer is:b
Ecell=0.059[pHa−pHc+12.(pH2)(pH2)c]
=0.059[0−0+12log(p_(1))/(p_(2))]<br>=(0.059)/(2)log p1p2=RT2Fln.p1p2
Similar Questions
The oxidation potential of a hydrogen electrode at pH=10 and PH2=1 is
View SolutionA cell contains two hydrogen electrodes. The negative electrode is in contact with a solution of 10−6M hydrogen ions. The EMF of the cell is 0.118V at 25∘C. Calculate the concentration of hydrogen ions at the positive electrode.
View Solution
Knowledge Check
EMF of hydrogen electrode in term of pH is (at 1 atm pressure).
AEH2=RTF×pHBEH2=RTF1pHCEH2=2.303RTFpHDEH2=0.0591pHThe emf of a cell is the ratio of the difference in
Acharge in coulombs to the electrical potential energy in joulesBelectrical potential energy in joules to the charge in coulombsCelectrical potential energy in joules to the resistanceDelectrical potential energy in joules to the timeEMF of hydrogen electrode in terms of pH is (at 1 atm pressure)
AEH2=RTF×pHBEH2=RTF=1pHCEH2=2.303RTFpHDEH2=−0.591pH
EMF of hydrogen electrode in term of pH is (at 1 atm pressure).
View SolutionEmf of hydrogen electrode in tern of pH is (at 1 atm pressure):
View SolutionThe half cell potential of a hydrogen electrode at pH = 10 will be
View SolutionFor the following cell with gas electrodes at p1andp2 as shown:
PtCl2atP1|HCl|1MPt(Cl2)atP2 cell reaction is spontaneous if :
View SolutionWhen zinc electrode and calomel electrode are coupled to form a cell , the cell emf is given as
View Solution

