Arrange the following compounds in the order of increasing conductance :HCl,LiCl,NaCl,KCl.
A
LiCl>NaCl>KCl
B
KCl>NaCl>LiCl
C
NaCl>KCl>LiCl
D
LiCl>KCl>NaCl
Video Solution
Text Solution
The correct Answer is:b
Refer Section and Illustration
|
Topper's Solved these Questions
- ELECTROCHEMISTRYBOOK - CENGAGE CHEMISTRYCHAPTER - ELECTROCHEMISTRYEXERCISE - Archieves Fill In The Blanks
4 Videos
- ELECTROCHEMISTRYBOOK - CENGAGE CHEMISTRYCHAPTER - ELECTROCHEMISTRYEXERCISE - Archieves True/False
1 Video
- ELECTROCHEMISTRYBOOK - CENGAGE CHEMISTRYCHAPTER - ELECTROCHEMISTRYEXERCISE - Archieves Multiple Correct Ansers
2 Videos
- D AND F BLOCK ELEMENTSBOOK - CENGAGE CHEMISTRYCHAPTER - D AND F BLOCK ELEMENTSEXERCISE - Archives Subjective
29 Videos
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBOOK - CENGAGE CHEMISTRYCHAPTER - GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSEXERCISE - Archives (Subjective)
14 Videos
Similar Questions
Knowledge Check
Arrange the following in the order of increasing entropy:
A1molofH2O(s)at0∘C and 1atm pressureB1molofH2O(s)at0∘C and 0.8atmpressureC1molofH2O(l)at25∘C and 1atm pressureD1molofH2O(l)at25∘C and 0.8atm pressureArrange the following acid in order of increasing acidity
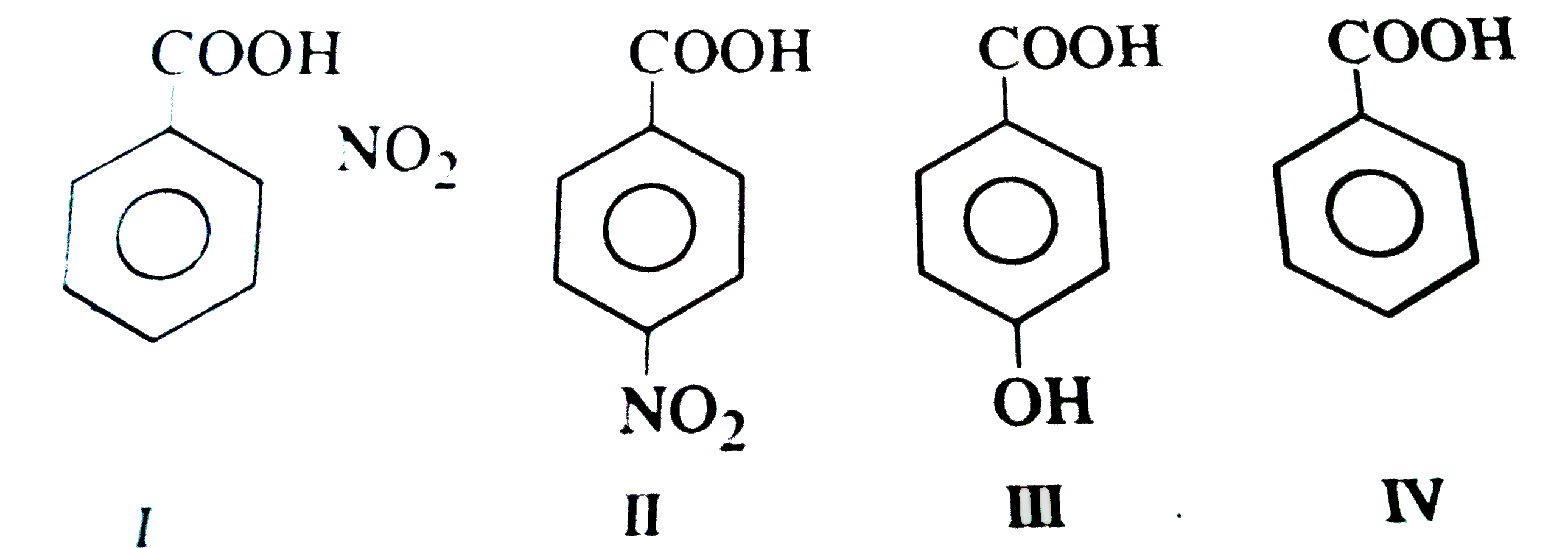 AI>II>III>IVBII>III>I>IVCIII>II>IV>IDIII>IV>II>I
AI>II>III>IVBII>III>I>IVCIII>II>IV>IDIII>IV>II>IArrange the following compounds in increasing order of acidity :
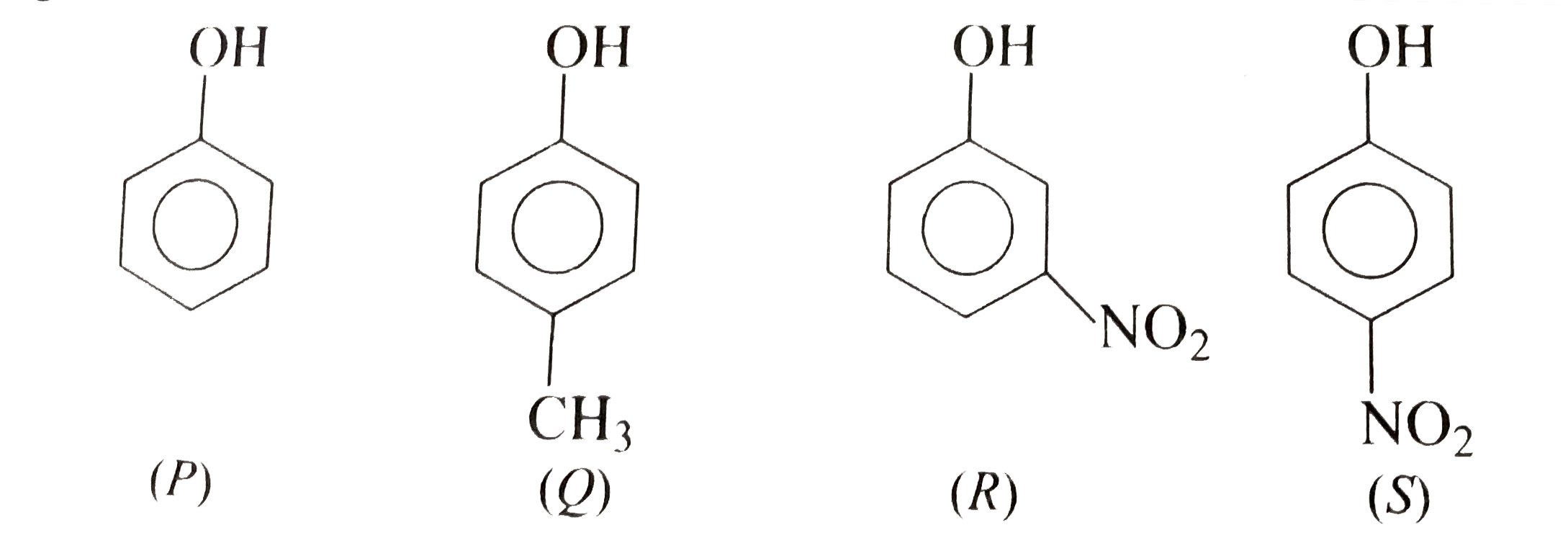 AP>Q>R>SBS>Q>R>PCS>R>P>QDR>S>R>P
AP>Q>R>SBS>Q>R>PCS>R>P>QDR>S>R>P
Arrange the following compounds in increasing order of basicity :
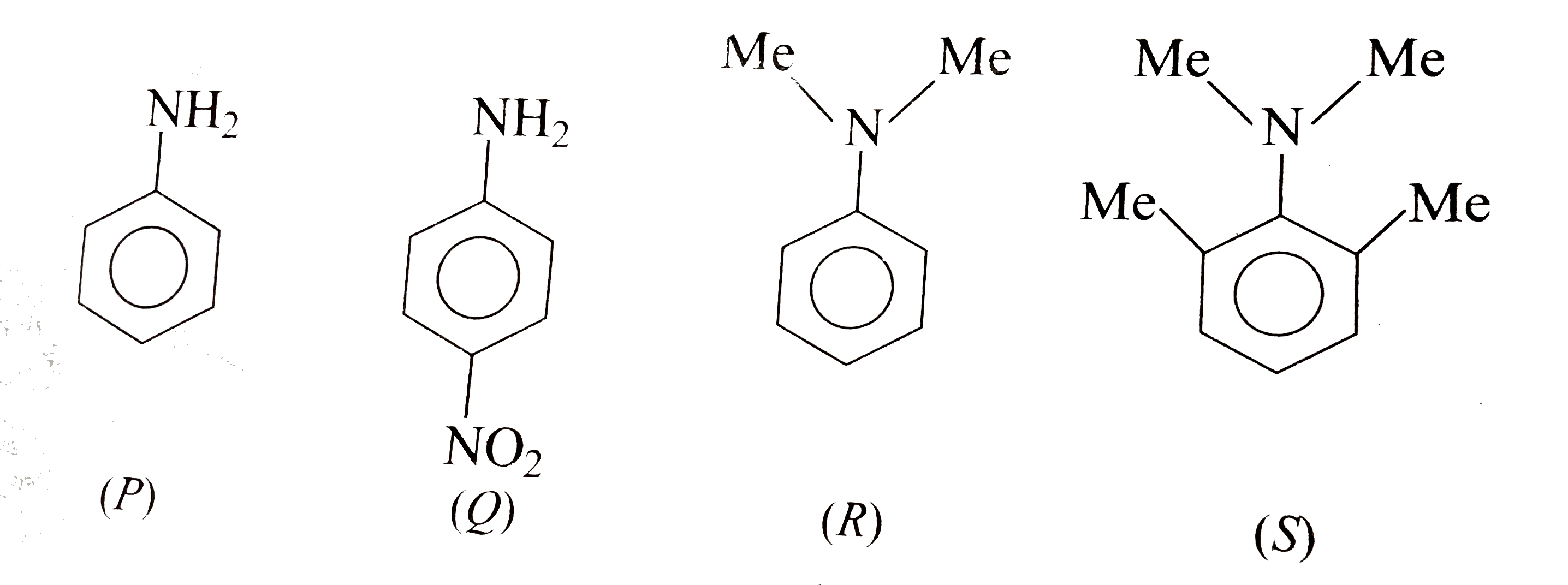
View SolutionArrange the following in the order of increasing ionization potential
View SolutionArrange the following compounds in increasing order of acid strength
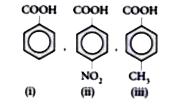
View SolutionArrange the following compounds in the increasing order of their melting point,
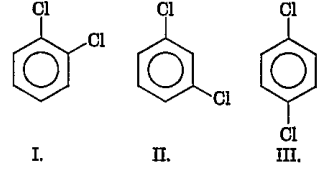
View SolutionArrange the following compounds in increasing order of their boiling
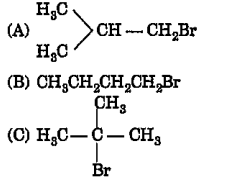
View Solution