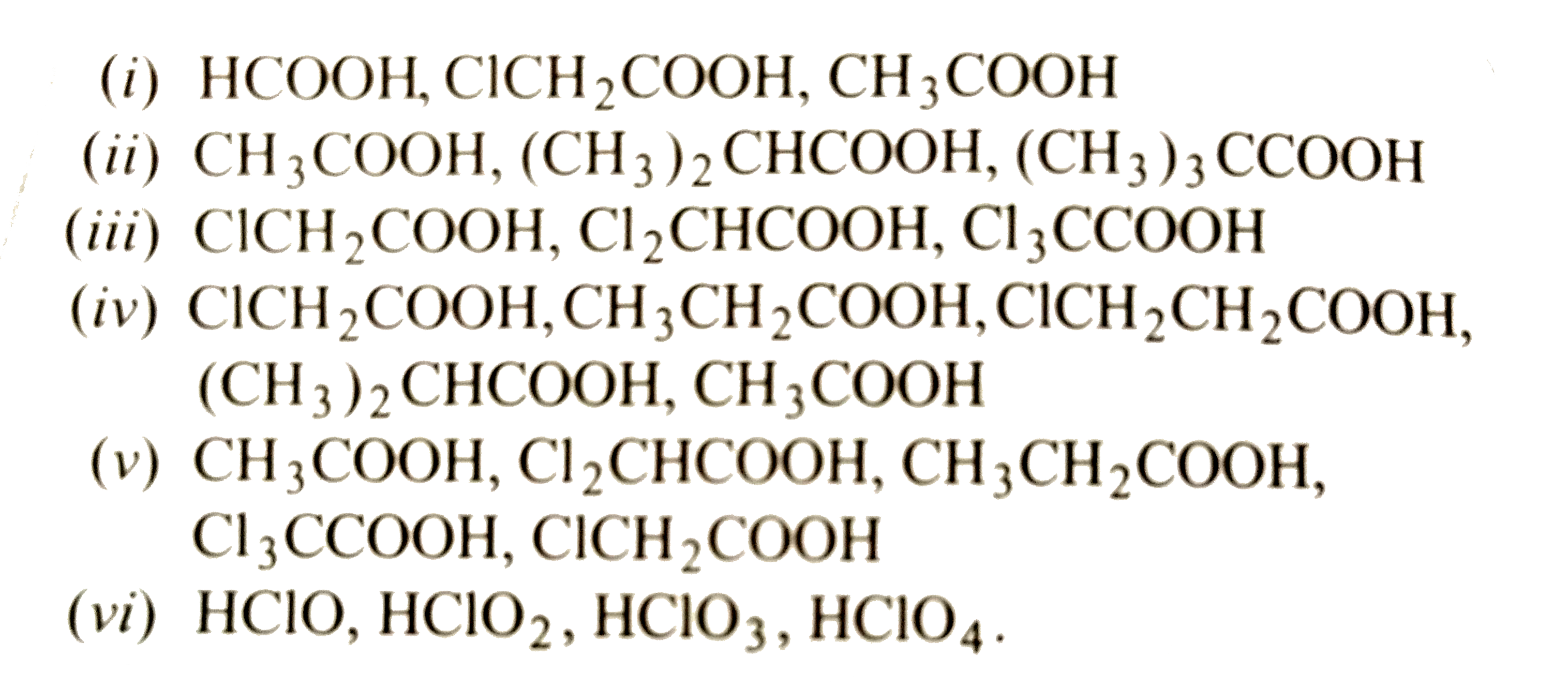Arrange the following in the order of increasing ionization potential
A
Mg<A1<Si<P
B
A1<Si<P<Mg
C
Si<P<Mg<A1
D
A1<Mg<Si<P
Video Solution
Text Solution
The correct Answer is:D
These elements are members of the 3rd period. In general ionization potential increases across the period on account of the increase of nuclear charged. But A1had unusually low ionization potential relative to Mg as the electron ionized in A1 is 3p1 while in Mg, it is 3s2. For a given value of n, the case of removel of electron is
s>p>d>f
The correct order of ionization enthalpy is
A1(577kJmol−1)<Mg(738)<Si(786)<P(1012)
|
Similar Questions
Knowledge Check
Arrange the following in the order of increasing entropy:
A1molofH2O(s)at0∘C and 1atm pressureB1molofH2O(s)at0∘C and 0.8atmpressureC1molofH2O(l)at25∘C and 1atm pressureD1molofH2O(l)at25∘C and 0.8atm pressureArrange the following acid in order of increasing acidity
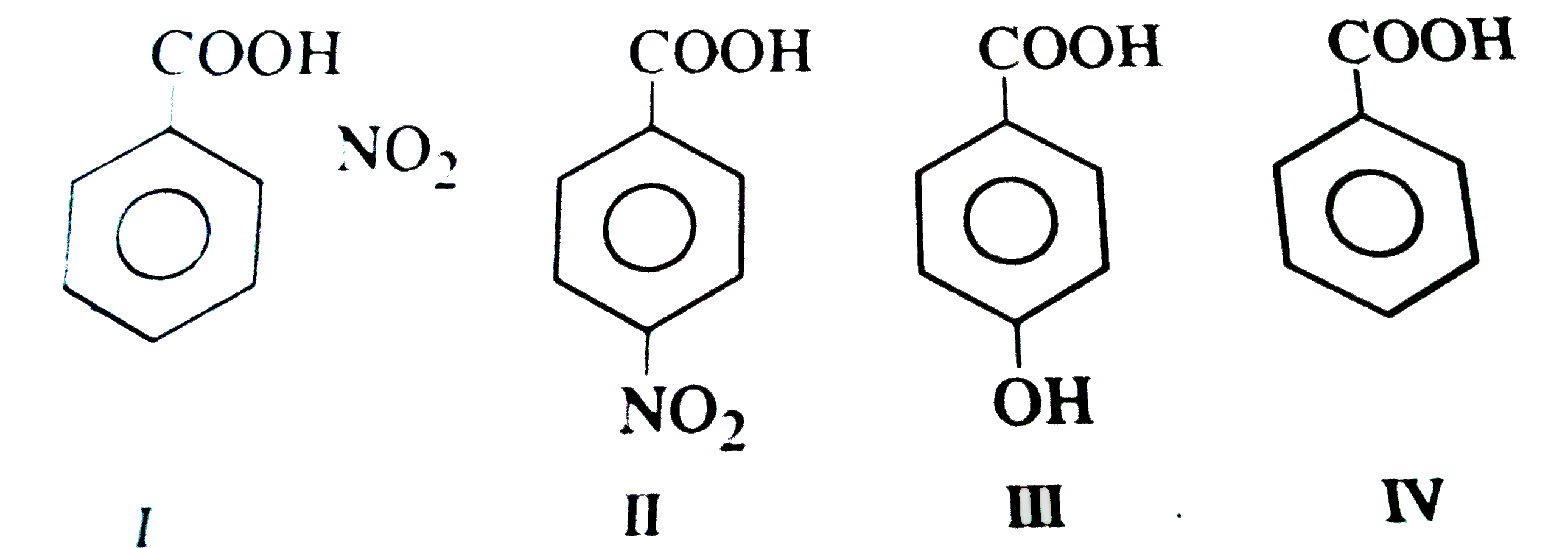 AI>II>III>IVBII>III>I>IVCIII>II>IV>IDIII>IV>II>I
AI>II>III>IVBII>III>I>IVCIII>II>IV>IDIII>IV>II>IArrange the following amines in the order of increasing basicity:
A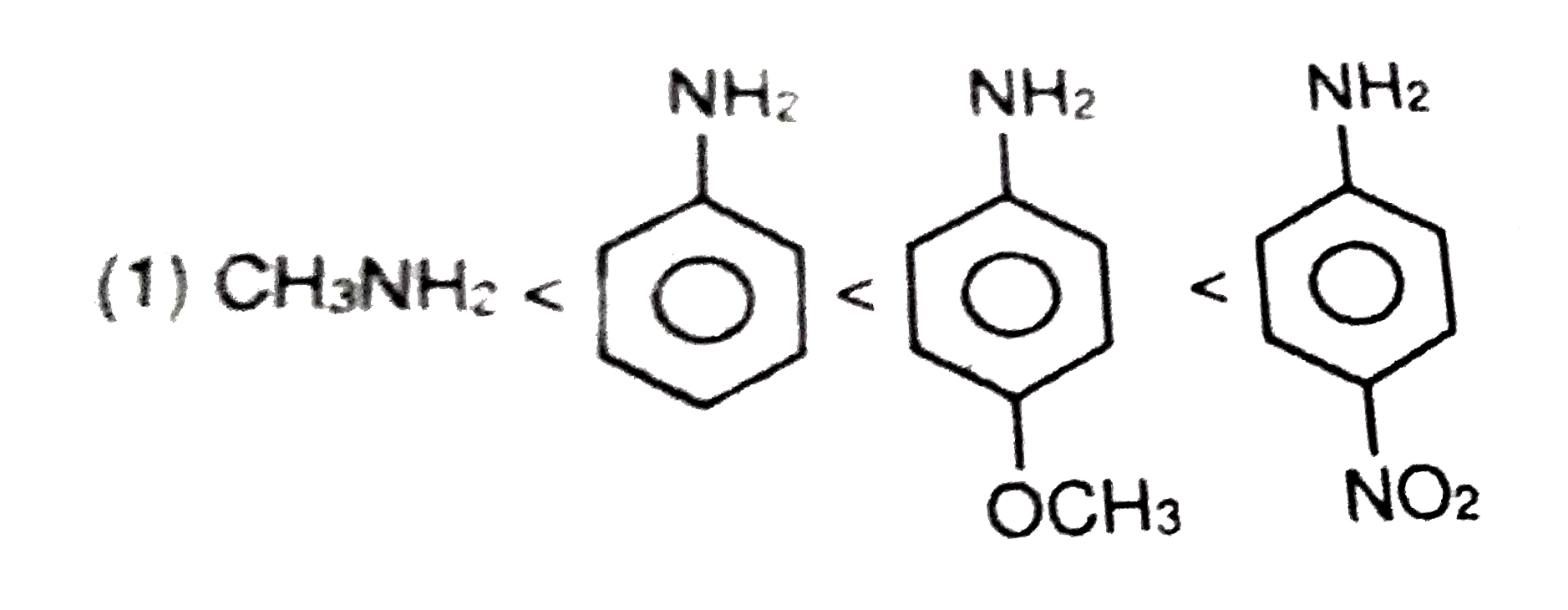 B
B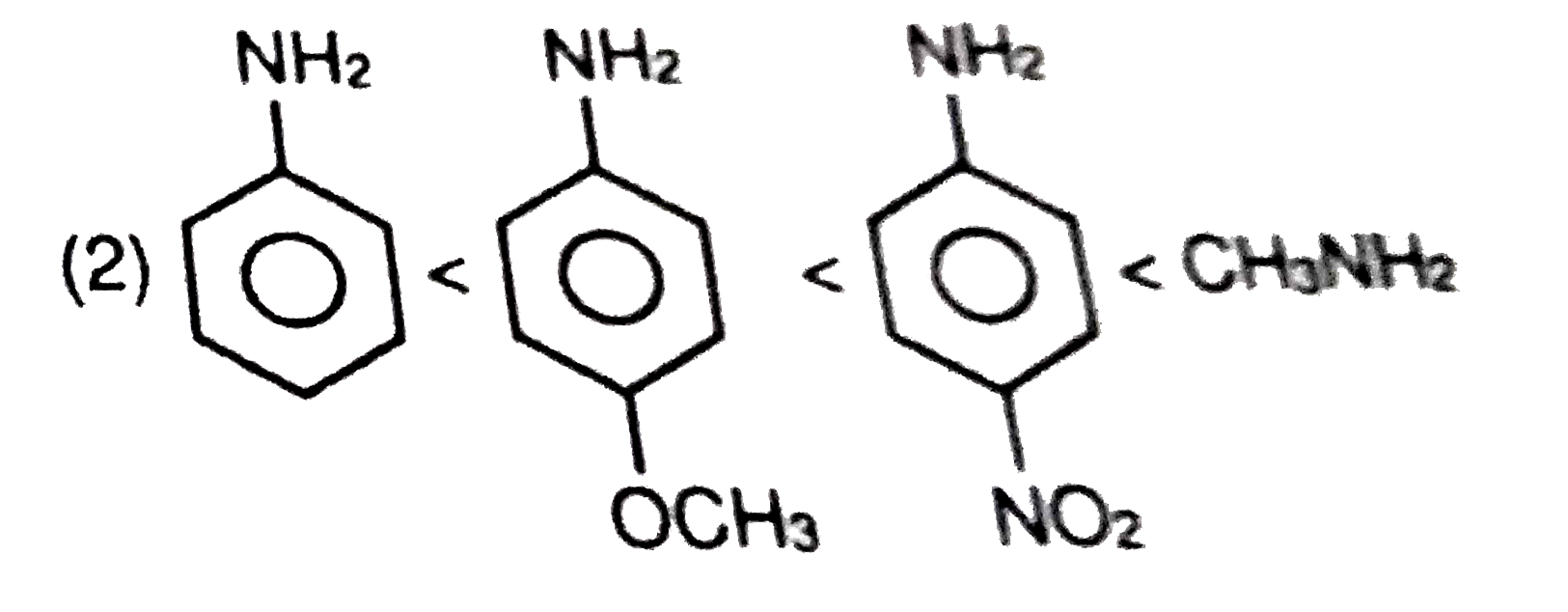 C
C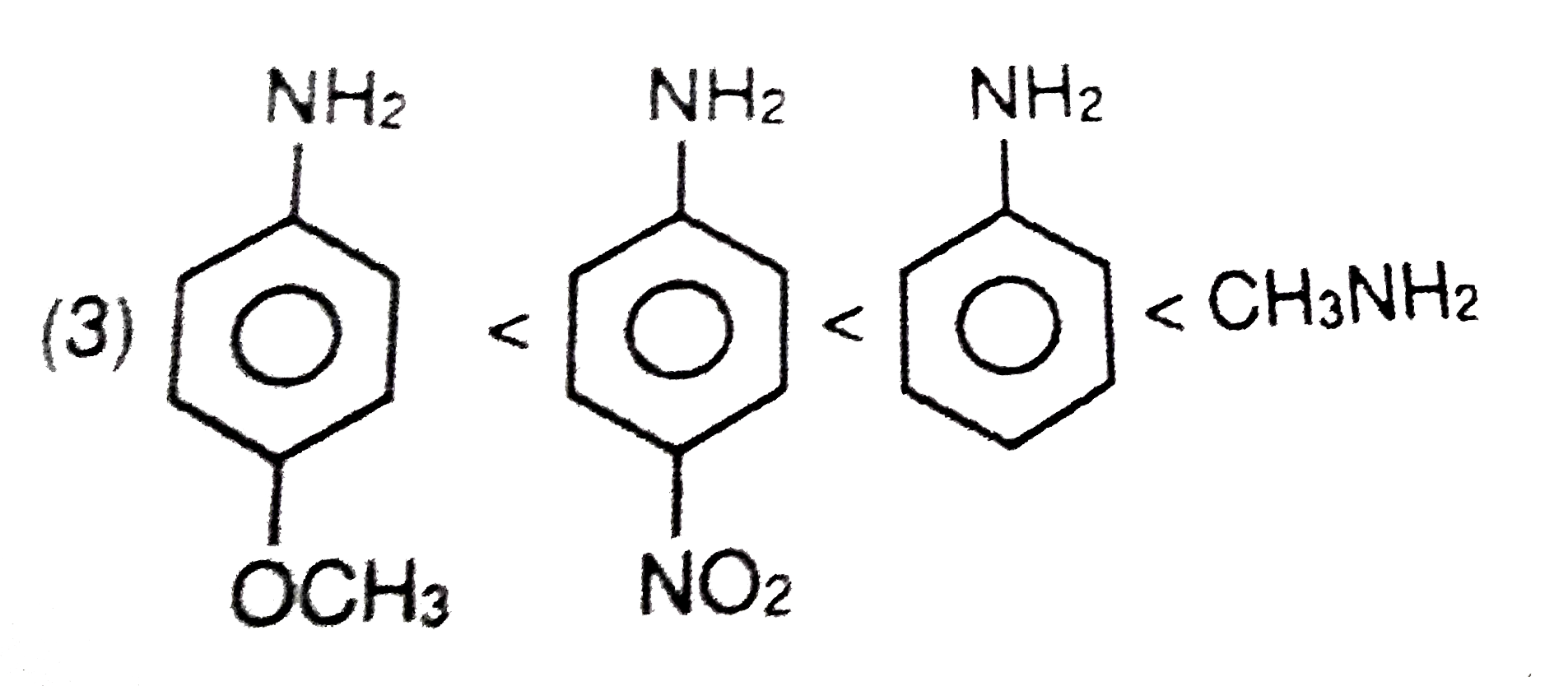 D
D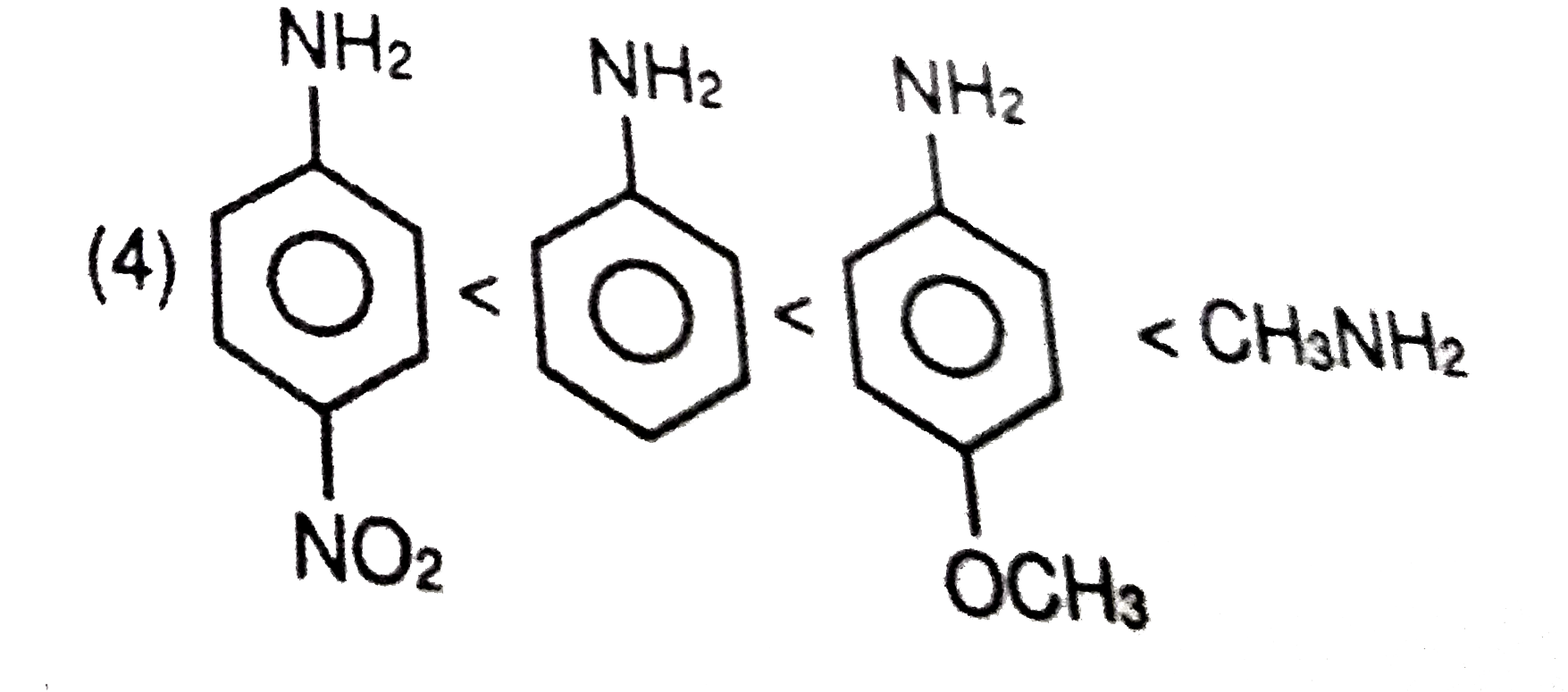
Arrange the following in order of increasing basic strength.
View Solution.Arrange the following in increasing order of ionization enthalpy: B,C,N,O
View SolutionArrange the following amines in the order of increasing basicity.
View SolutionArrange the following amines in the order of increasing basicity :-
View SolutionArrange the following elements in order of their increasing ionization energies: O, S, Se, Te, Po
View Solution