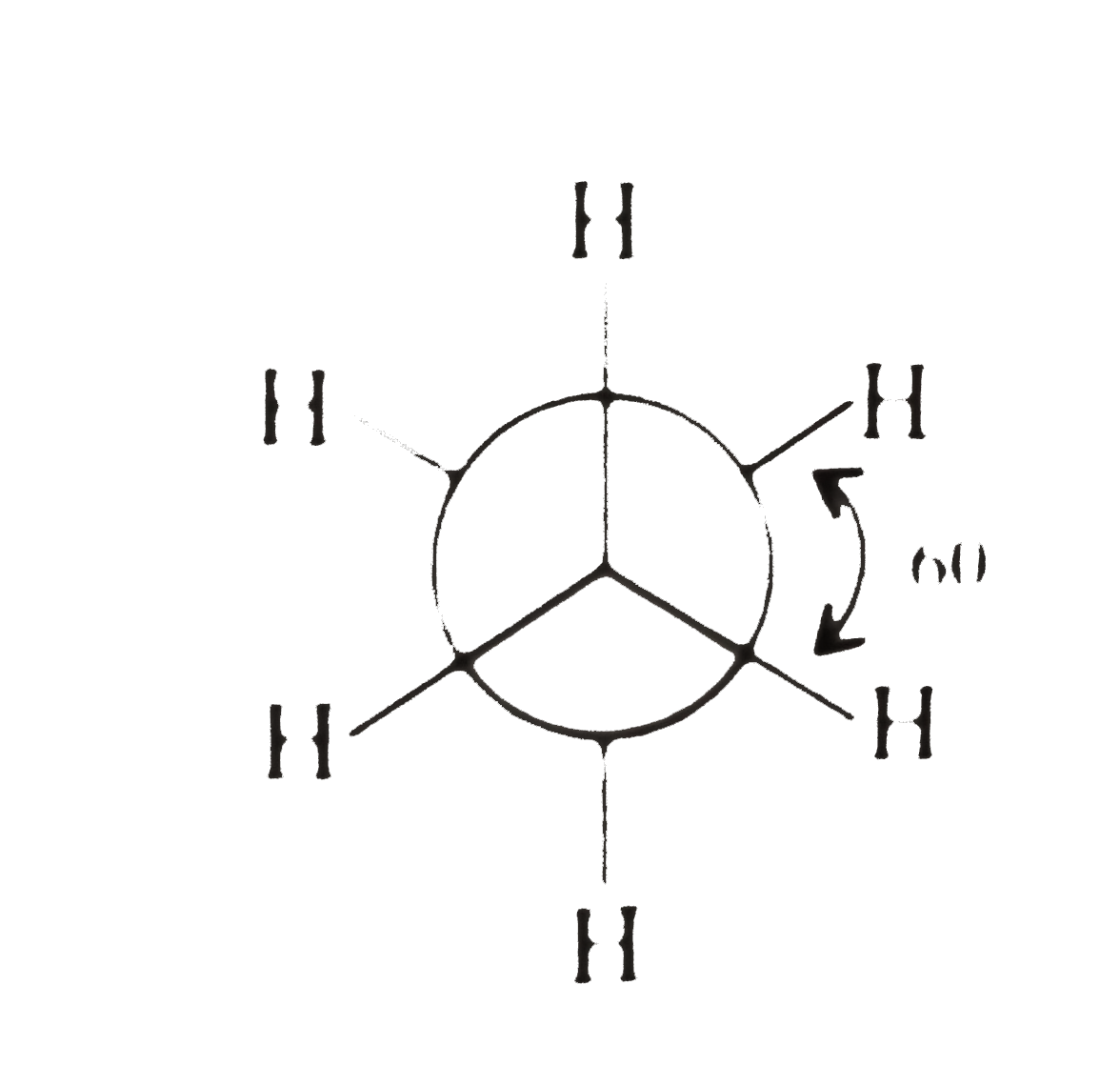
In the above compound, C2 is rotated clockwise 120∘ about C2−C3 bond. The resulting conformer is:
Partially eclipsed
Eclipsed
Gauche
Staggered
The correct Answer is:C
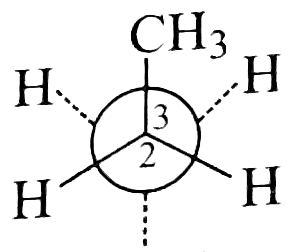
here when C2 is rotated clockwise 120∘ about C2−C3 bond, the resulting conformer is Gauche conformer.

Topper's Solved these Questions
- ISOMERISMBOOK - CENGAGE CHEMISTRYCHAPTER - ISOMERISMEXERCISE - Assertion-Reasoning Type (Exercise)
5 Videos
- ISOMERISMBOOK - CENGAGE CHEMISTRYCHAPTER - ISOMERISMEXERCISE - Fill in the blanks (Exercise)
3 Videos
- ISOMERISMBOOK - CENGAGE CHEMISTRYCHAPTER - ISOMERISMEXERCISE - Single correct answer type
13 Videos
- IONIC EQUILIBRIUMBOOK - CENGAGE CHEMISTRYCHAPTER - IONIC EQUILIBRIUMEXERCISE - Archives Subjective
28 Videos
- NCERT BASED EXERCISEBOOK - CENGAGE CHEMISTRYCHAPTER - NCERT BASED EXERCISEEXERCISE - Chemical Equilibrium
72 Videos
Similar Questions
Assertion (A): Metamers can also be position or chain isomers.
Reason (R ): Tautomerism was introduced by C.P. Laar to explain the chemical reactivity of a substance according to two possible structrues.
View SolutionIsomers which are …… mirror images are knows as ….. (superimposable, non-superimposable, enantiomers, diastereomers, epimers)
View SolutionWhich of the following will not be able to show optical isomerism (enantiomerism)?
View SolutionHow much moles of sodium propionate should be added to 1L of an aqueous solution containing 0.020mol of propionic acid to obtain a buffer solution of pH4.75? What will be the pH if 0.010mol of HCI is dissolved in the above buffer solution. Compare the last pH value with the pHof0.010MHCI solution. Dissociation constant of propionic acid, Ka, at 25∘C is 1.34×10−5.
View SolutionA liquid is in equilibrium with its vapour in a sealed container at a fixed temperature. The volume of the container is suddenly increased.
a. what is the initial effect of the change on vapour pressure?
b. How do rates of evaporation and condensation change initially?
c. What happens when equilibrium is restored finally and what will be the final vapour pressure?
View SolutionIn the given conformation, if C2 is rotate about C2−C3 bond anticlockwise by an angle of 120∘ then the conformation obtained is

View SolutionHow many extreme conformations can be drawn for n -butane if we consider rotation about the C_(2) and C_(3) bonds?
View SolutionC_(2) is rotated anti-clockwise 120^(@) about C_(2)-C_(3) bond. The resulting conformer is
View SolutionWhich of the Newman projections shown below represents the most stable conformation about the C1−C2 bond of 1-iodo-2-methyl propance?
View SolutionMost stable conformation of n-pentane is : ( Consider C_(2)-C_(3) bond rotation )
View SolutionHO−CH2−CH2−O∣∣C−H (3) (2) (1)
Which conformer of above compound id most stable (consider conformer across (C2−C3)
View SolutionWhich conformational state of n-butane lies in higher energy state when rotated along C_(2)-C_(3) bond?
View SolutionNewman projection of Butane is given, C-2 is rotated by 120^(º) along C_(2)-C_(3) bond in anticloclwise direction, the conformation formed is :
View Solutionदिए गए संरूपण में C2 को C2−C3 बंध के परित: दक्षिणावर्त 120∘ के कौन पर घुमाया जाता है। प्राप्त संरूपण है
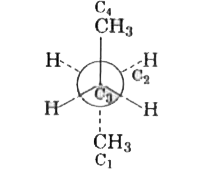
View SolutionIn the given conformation C2 is rotated about C2−C3 bond by 120∘, then the conformation formed will be
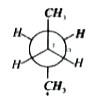
View Solution

