Newman projection of Butane is given, C-2 is rotated by 120^(º) along C2−C3 bond in anticloclwise direction, the conformation formed is :
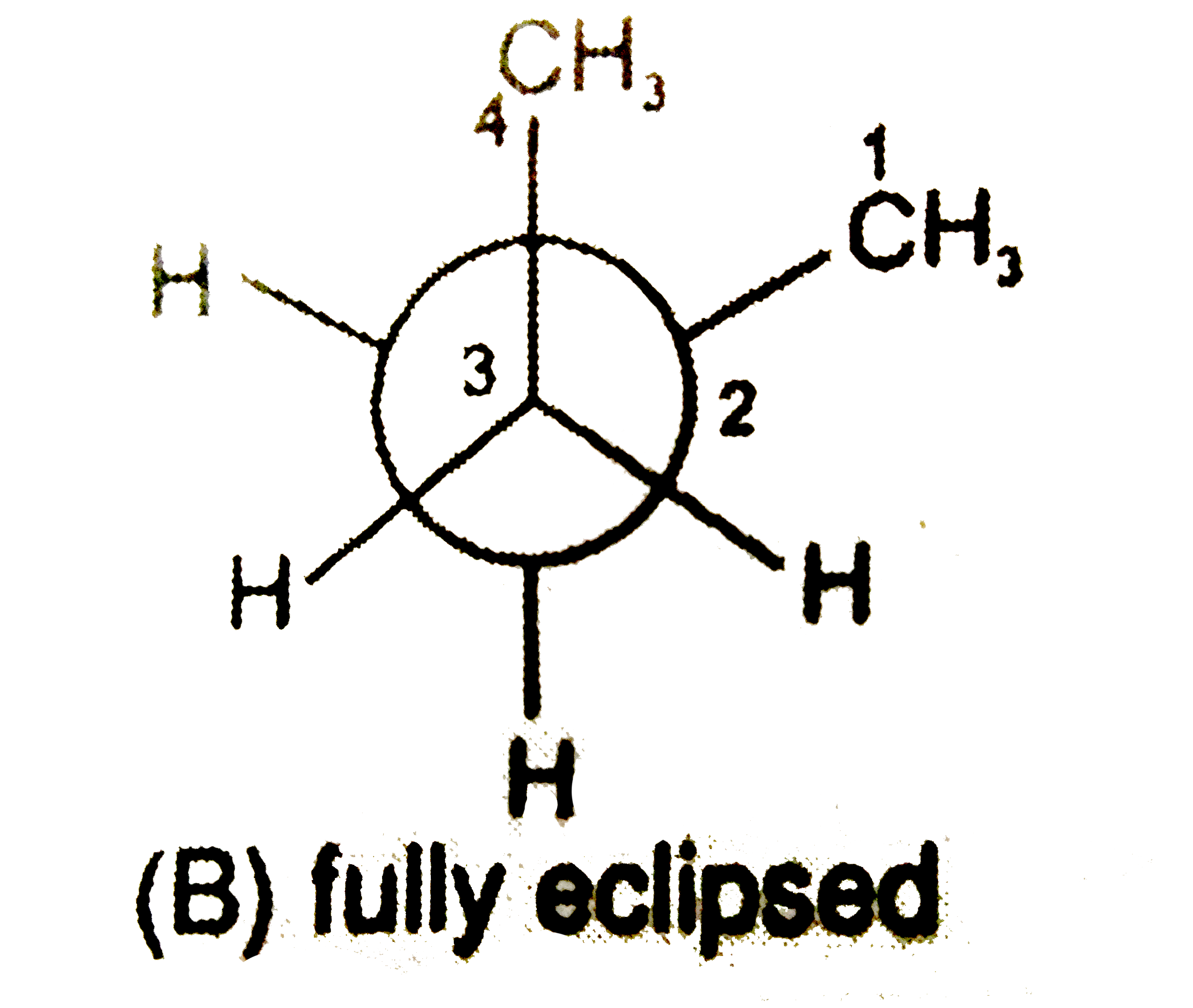
A
anti
B
fully eclipsed
C
gauche
D
partially eclipsed
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
In the given conformation , if C2 is rotated about C2−C3 bond anticlockwise by an angle of 120∘ then the conformation obtained is
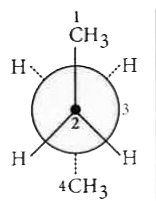 Afully eclipsed conformationBpartially eslipsed conformationCgauche conformationDstaggered conformation
Afully eclipsed conformationBpartially eslipsed conformationCgauche conformationDstaggered conformationIn the given conformation, if C2 is rotate about C2−C3 bond anticlockwise by an angle of 120∘ then the conformation obtained is
 Afully eclipsed conformationBpartially eclipsed conformationCgauche conformationDstaggered conformation
Afully eclipsed conformationBpartially eclipsed conformationCgauche conformationDstaggered conformationIn the given conformation C2 is rotated about C2−C3 bond anticlockwise by an angle of 120∘ then the conformation obtained is
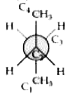 Afully eclipsed conformationBpartially eclipsed conformationCgauche conformationDstaggered conformation.
Afully eclipsed conformationBpartially eclipsed conformationCgauche conformationDstaggered conformation.
A new C-C bond is formed in-
View SolutionWhich conformational state of n-butane lies in higher energy state when rotated along C2−C3 bond?
View Solution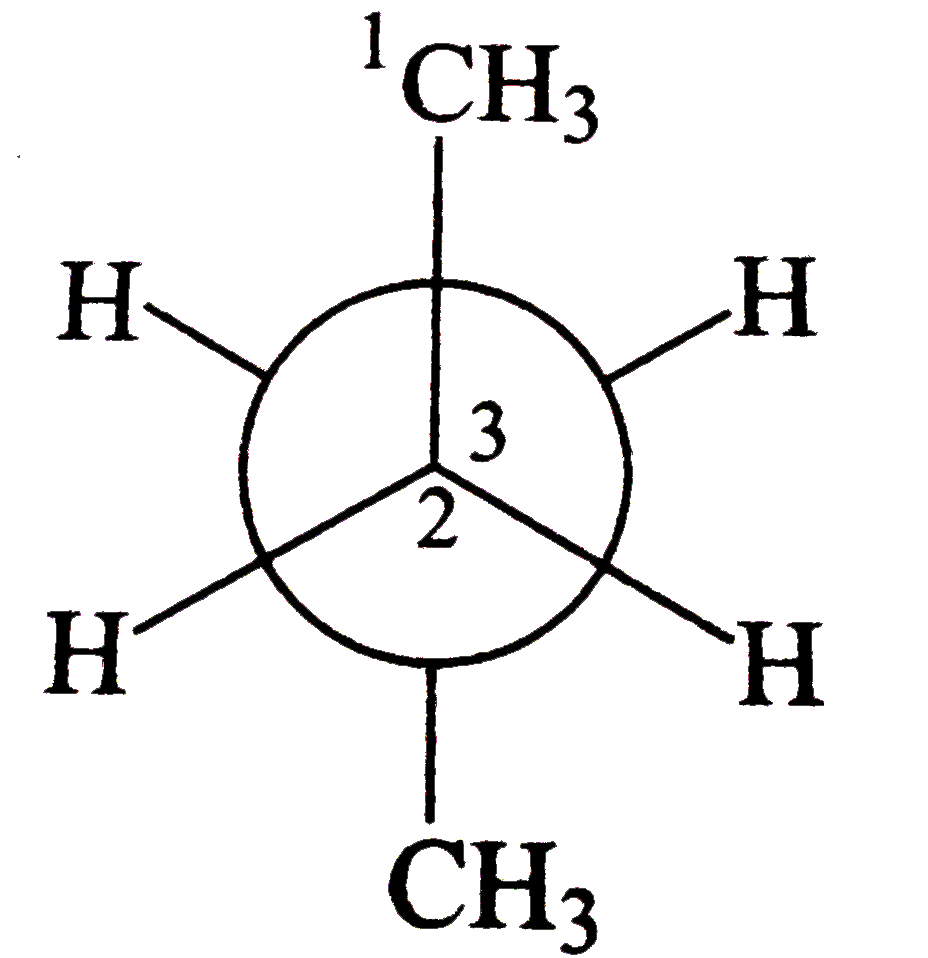
C2 is rotated anti-clockwise 120∘ about C2−C3 bond. The resulting conformer is
View Solution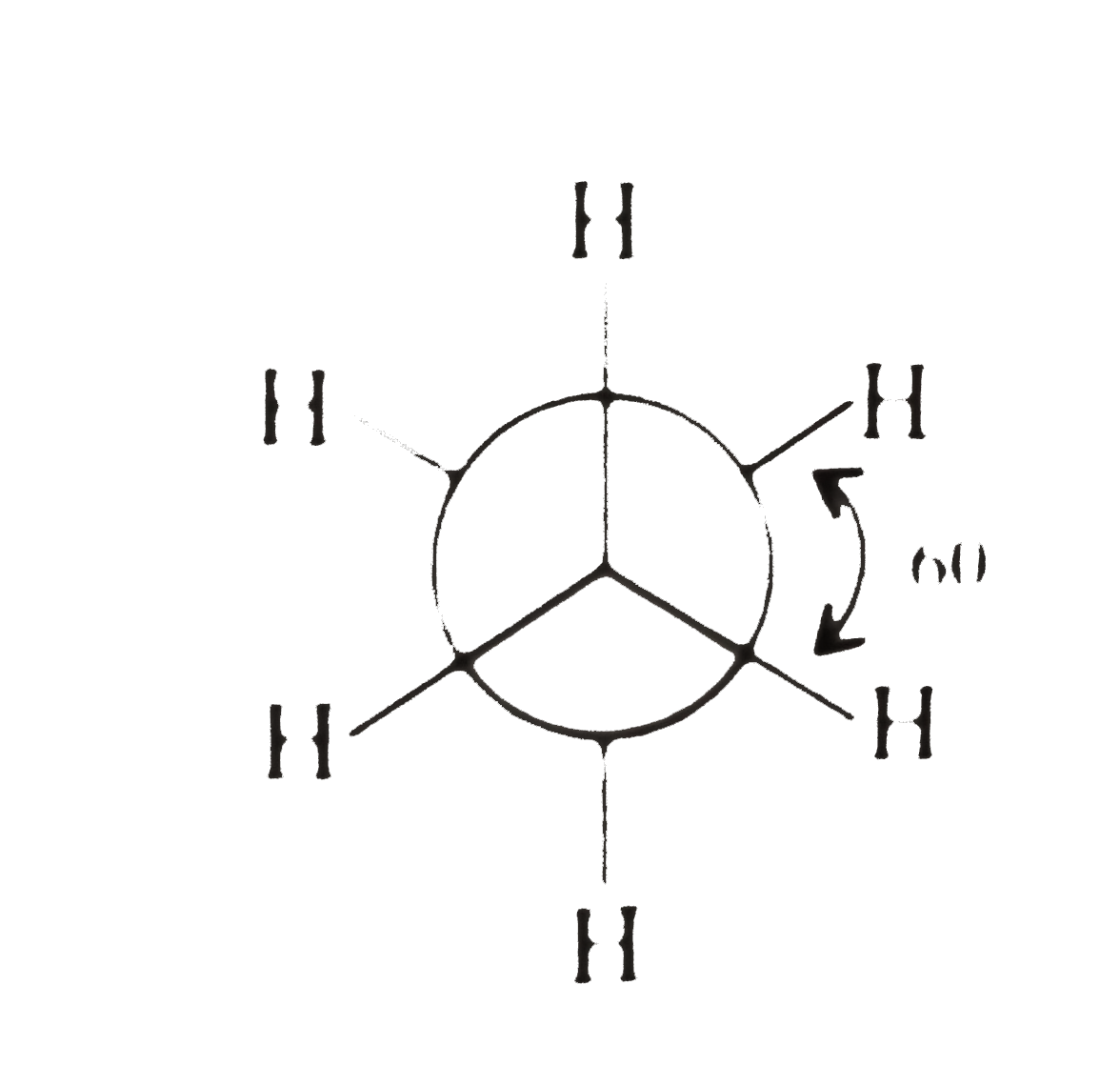
In the above compound, C2 is rotated clockwise 120∘ about C2−C3 bond. The resulting conformer is:
View SolutionIn but-1, 3-diene the C2−C3 bond length is
View Solution

