How is the molar conductivity of strong electrolytes at zero concentration determined by graphical method? Why is this method not useful for weak electrolytes?
Text Solution
(1) Molar conductivity is the conductance of all the ions produced from one mole of an electrolyte. The molar conductivity of both, strong and weak electrolytes increases with dilution or the decrease in concentration of the electrolytic solution.
As the dilution increase , the dissociation of the electrolyte increases, hence the total number of ions increases, therefore, the molar conductivity increases.
(3) Molar conductivity ∧m is given by ∧m=kC=k×V where k is conductivity and C or V are concentration or dilution of the solution respectively.
(4) On dilution, the molar conductivity of strong electrolytes increase rapidly and approaches to a maximum limiting value at infinite dilution or zero concentration and respresented as ∧∞ or ∧0 or ∧0m .
(5) In case of weak electrolytes, the molar condutivity is low and condutivity is low and increase slowly in high concentration region but incerease rapidly at low concentration or high dilution since the extent of dissocitate inrease with dilution rapily.
For strong electrolytes, the linear variation of molar conductivity (Am) with the concentration is represented by Kohlrausch relation, ∧m=∧0−a√c where a is a constant .
∧0 values for strong electrolytes can be obtained by extrapolating the linear graph to zero concentration (or infinite dilution). However A, for the weak electrolytes cannot be obtained by this method, since the graph increases exponentially at very high dilution and does not intersect ∧m axis,
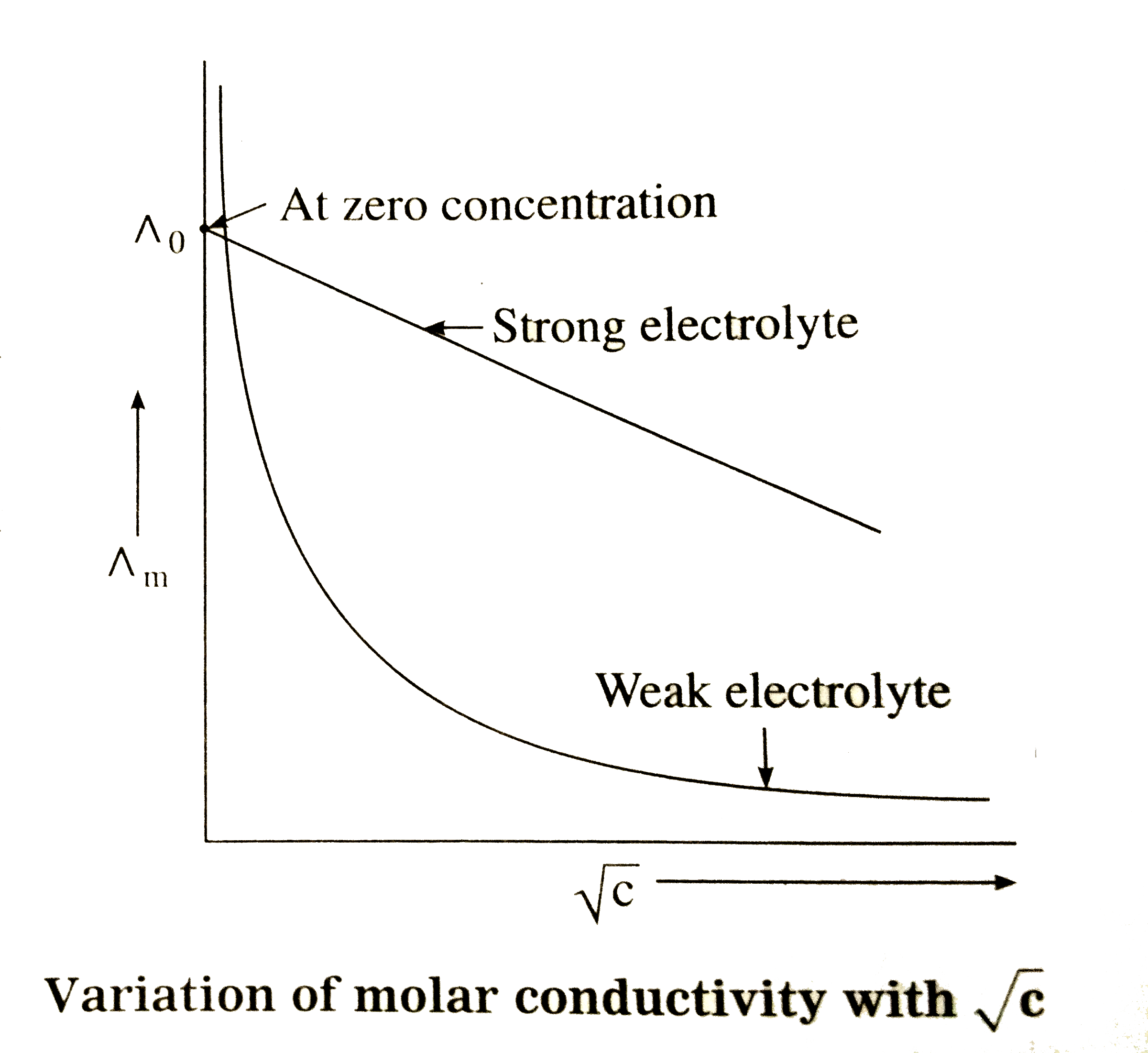
|
Similar Questions
Knowledge Check
The conductivity of a strong electrolyte:
Aincreseas on dilution slightlyBdecreases on dilutionCdoes not change with dilutionDdepends upon density of electrolyte itselfThe molecular conductivity of strong electrolyte
Aincreases lineraly with concentrationBincreases linearly with concentration in a linear fashionCdecreases lineraly with concentrationDdecrases with square root of concentration in a linear fashion.Conductivity of a strong electrolyte .
AIncreases on dilutionBDepends on densityCDecreases on dilutionDDoes not change considerable on dilution
How the weak and strong electrolytes are distinguished?
View SolutionResistivity is reciprocal of molar conductivity of electrolyte.
View SolutionThe conductivity of a strong electrolyte:
View SolutionThe equivalent conductance of strong electrolyte:
View SolutionThe plot of molar conductance vs √C in strong electrolyte , is
View Solution

