HCl can be prepared by
A
NaCl+H2SO4420K−−→
B
NaHSO4+NaCl820K−−→
C
NaNO3+H2SO4
D
both (a) and (b).
Video Solution
Text Solution
The correct Answer is:D
NaCl+H_(2)SO_(4)overset(420 K)rarrNaHSO_(4)+HCl<br>NaHSO2+NaCl823K−−→Na2SO4+HCl
|
Topper's Solved these Questions
- THE P-BLOCK ELEMENTS BOOK - NCERT FINGERTIPSCHAPTER - THE P-BLOCK ELEMENTS EXERCISE - Oxoacids Of Halogens
3 Videos
- THE P-BLOCK ELEMENTS BOOK - NCERT FINGERTIPSCHAPTER - THE P-BLOCK ELEMENTS EXERCISE - Interhalogen Compounds
2 Videos
- THE P-BLOCK ELEMENTS BOOK - NCERT FINGERTIPSCHAPTER - THE P-BLOCK ELEMENTS EXERCISE - Chlorine
4 Videos
- THE D- AND F- BLOCK ELEMENTS BOOK - NCERT FINGERTIPSCHAPTER - THE D- AND F- BLOCK ELEMENTS EXERCISE - Assertion And Reason
15 Videos
- THE SOLID STATE BOOK - NCERT FINGERTIPSCHAPTER - THE SOLID STATE EXERCISE - Assertion And Reason
15 Videos
Similar Questions
Knowledge Check
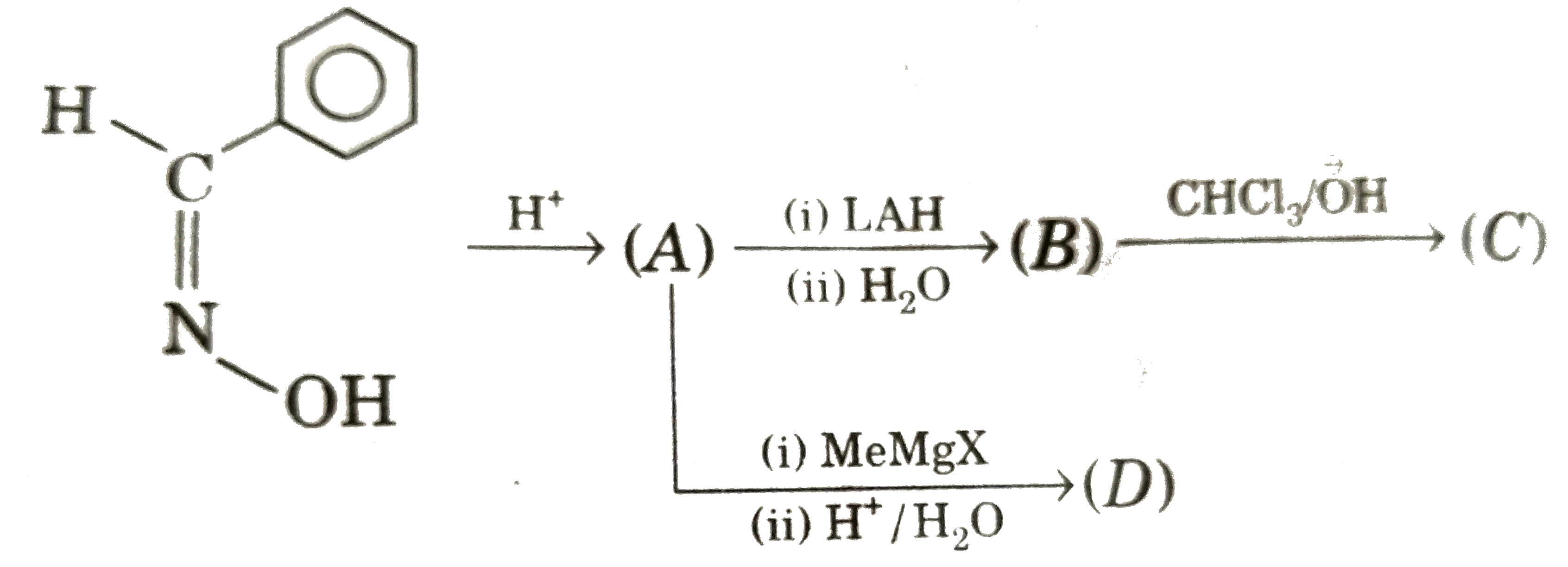
A can be prepared byAPh−C∣∣O−Cl+NH3→BPh−C∣∣O−Cl+NH3POCl3−−−→ΔC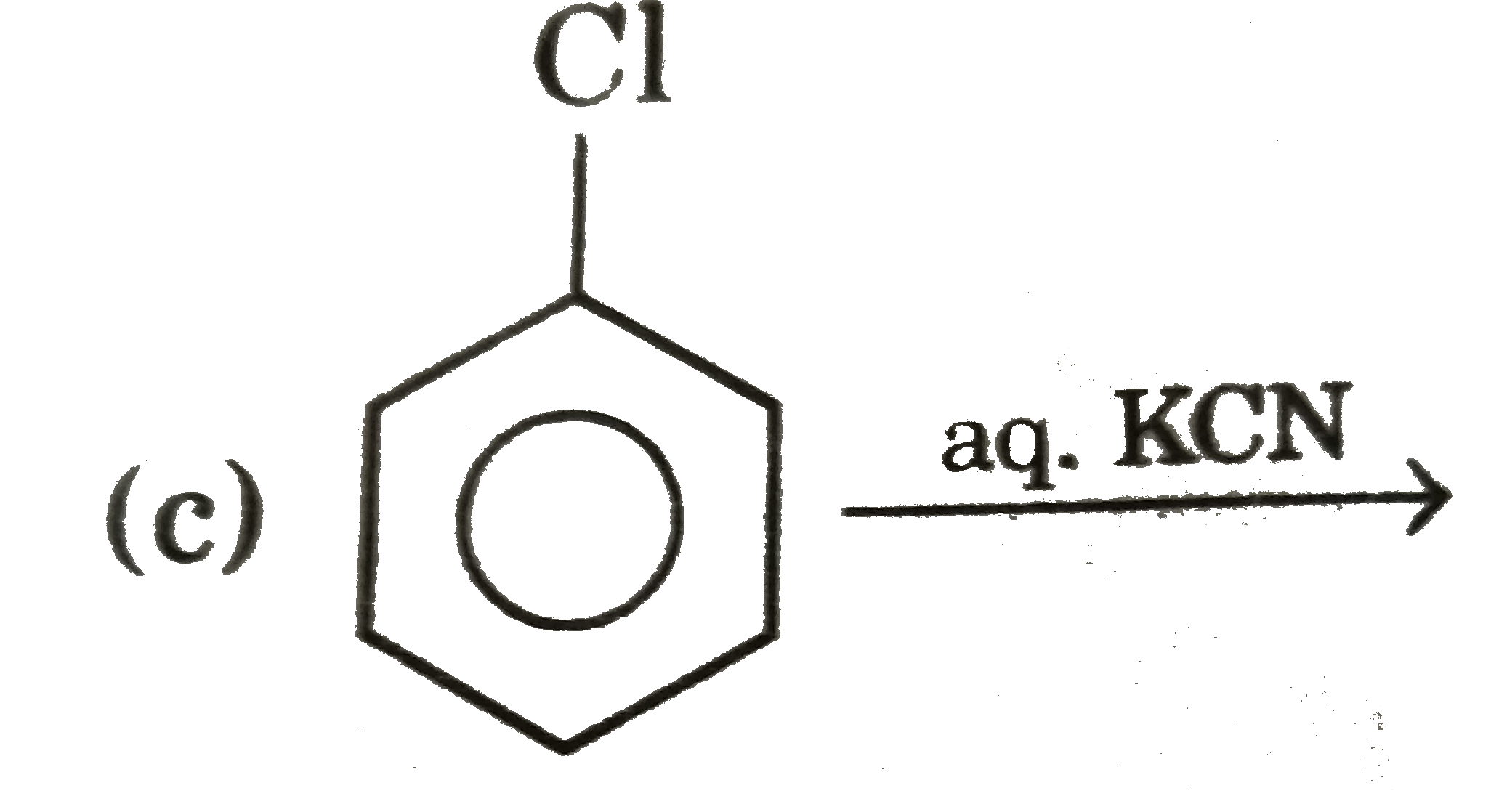 D
D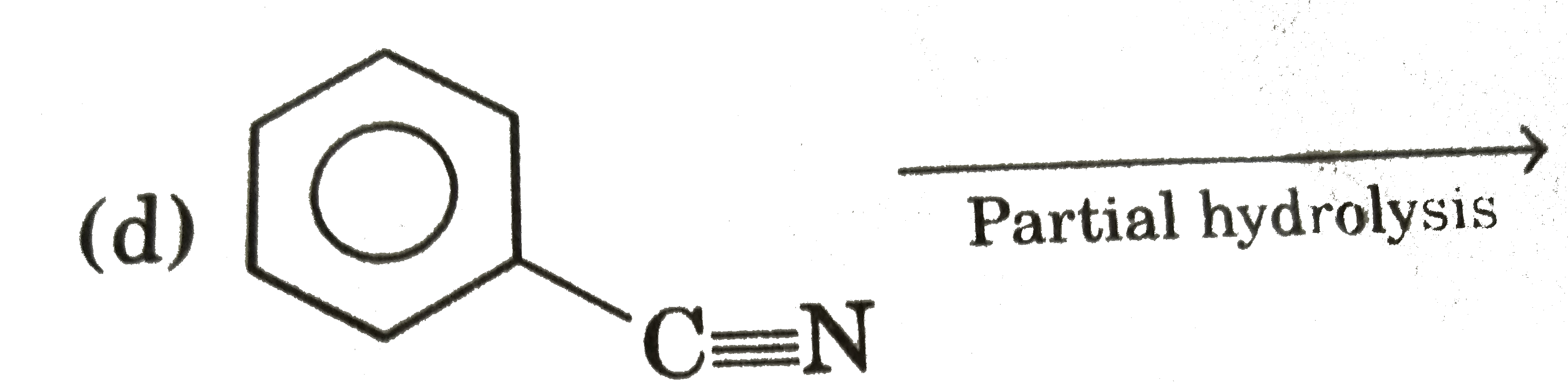
CO can be prepared by
Aheating HCOOH with conc. H2SO4Bheating K4[Fe(CN)6] with conc. H2SO4Cheating malonic acid with P2O5Dhydrolysis of Mn5C2Alcohols can be prepared by hydration of,
AalkanesBalkyl halidesCalkyl aminesDalkenes

