Choose the correct X and Y in the given reactions .
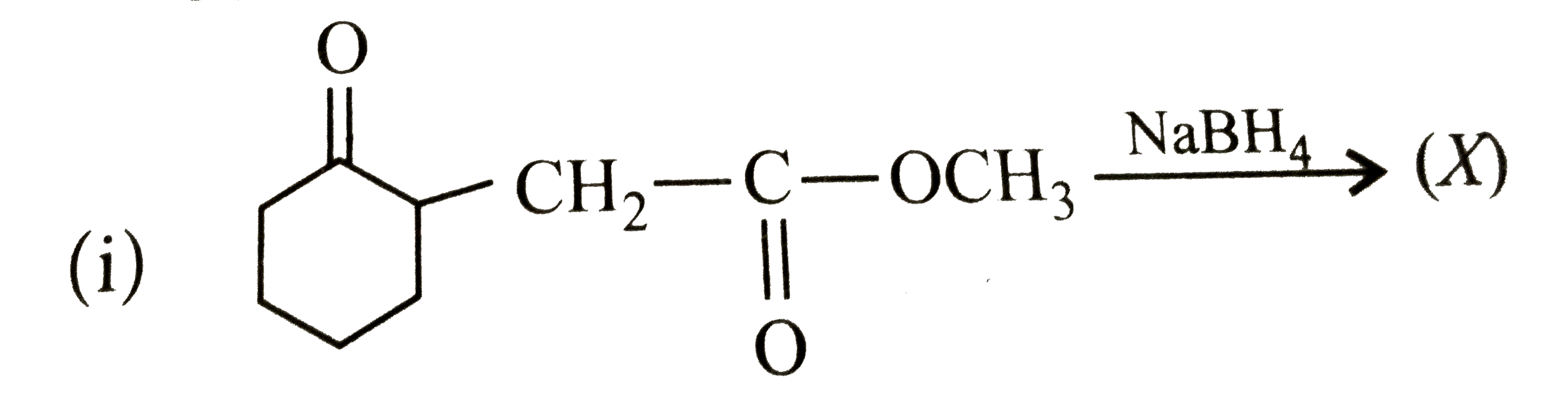
CH3CH2C∣CH3H−CHONaBH4−−−−→(Y)
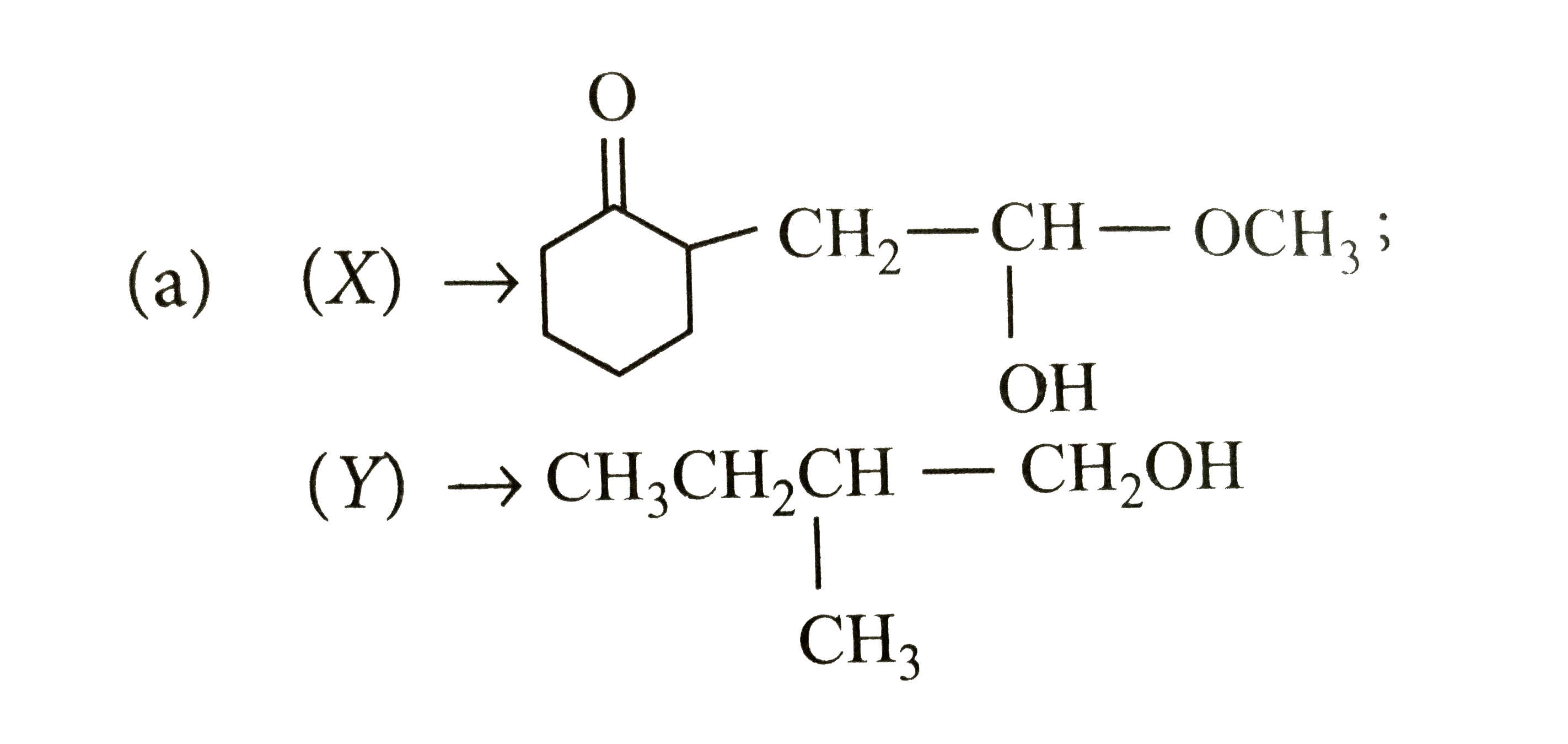
A
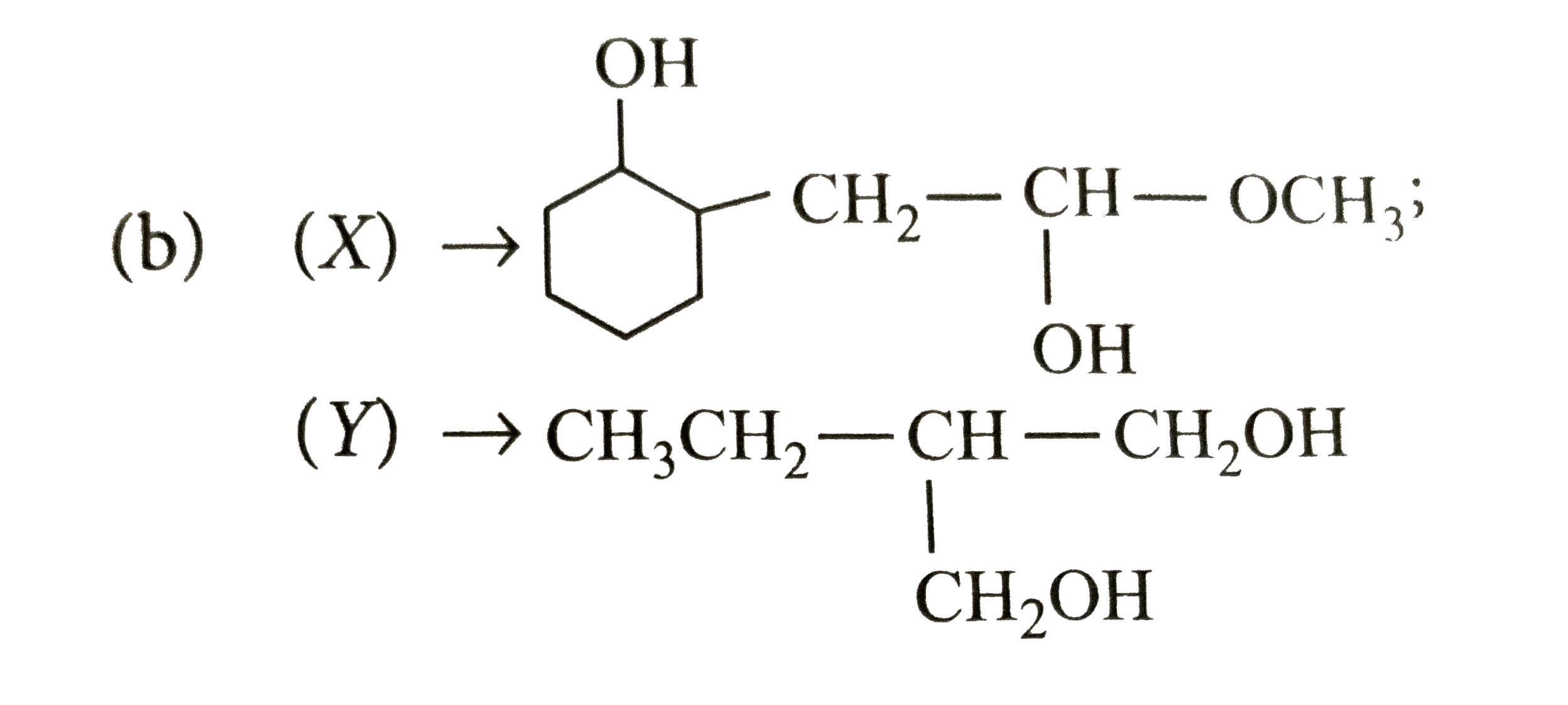
B
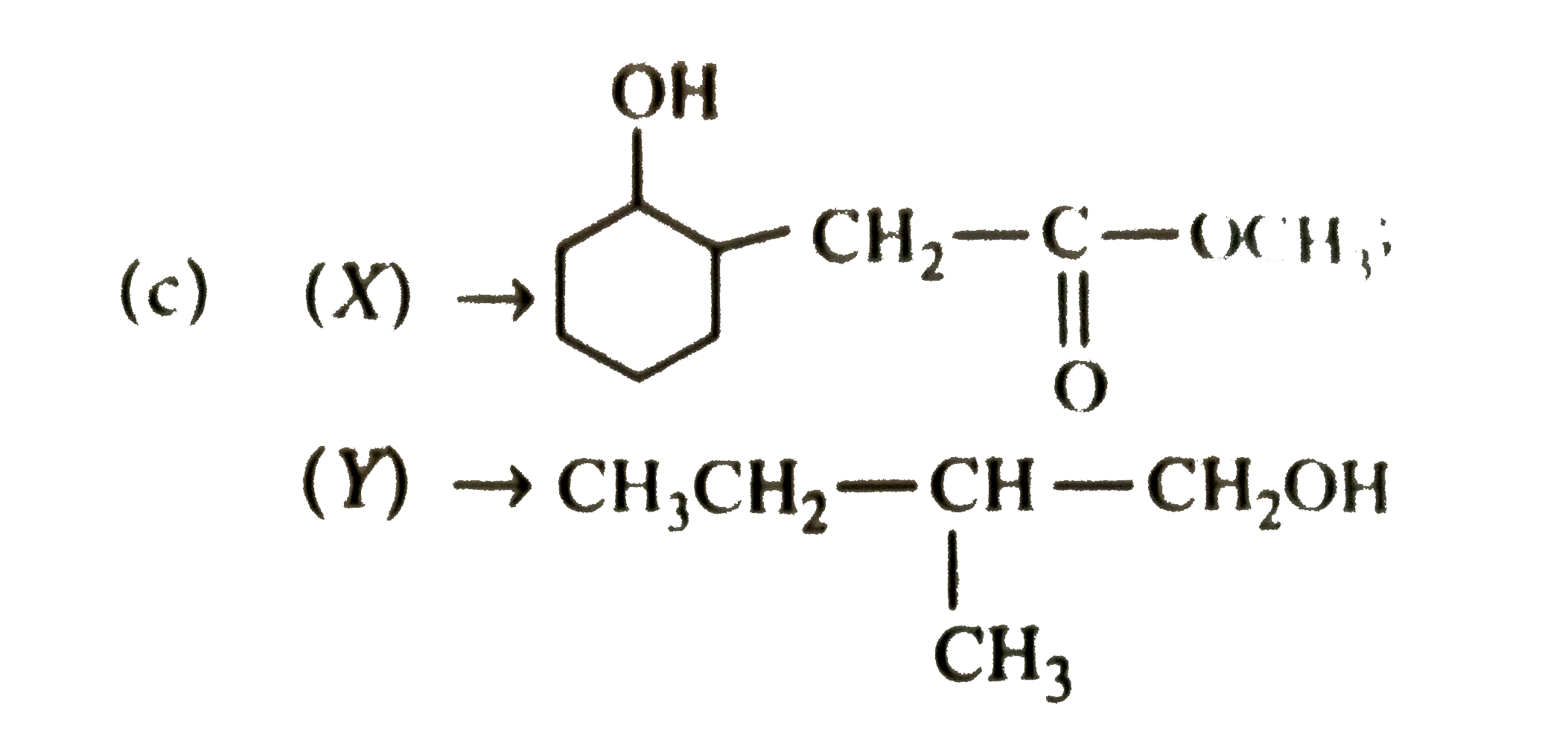
C
D
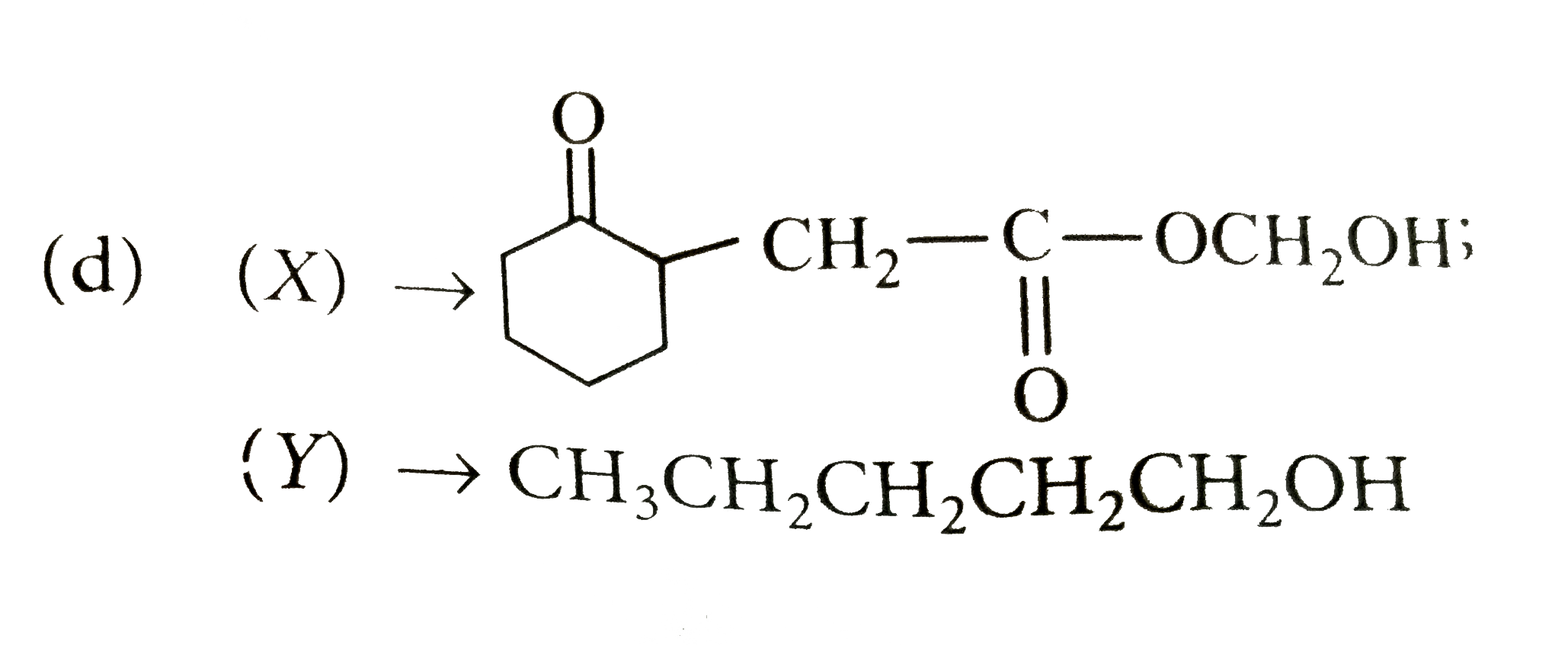
(Y)−CH3CH2CH2CH2CH2OH
Video Solution
Text Solution
The correct Answer is:C
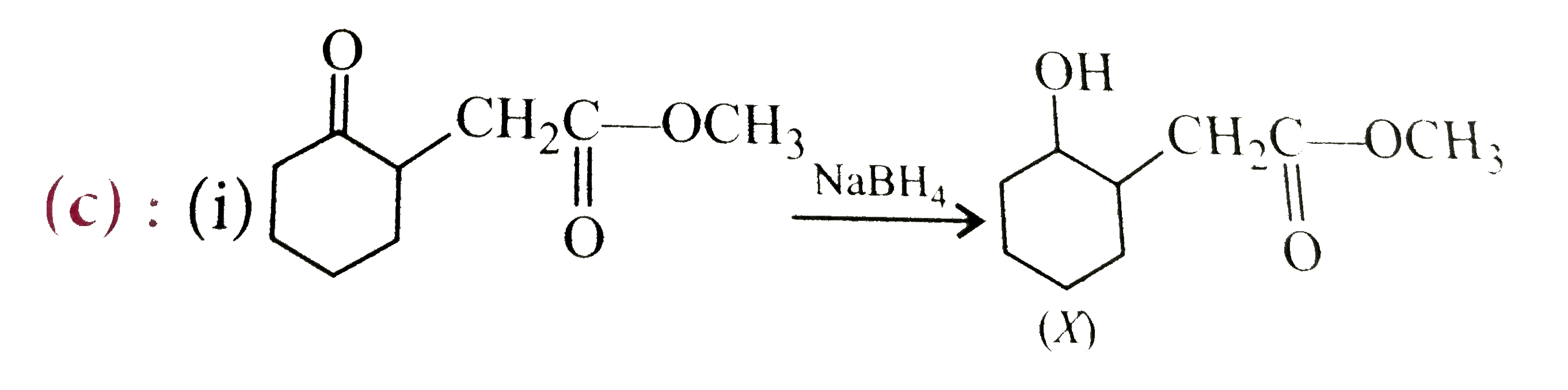
CH3CH2−CHCHO∣CH3NaBH4−−−−→CH3CH2CH∣CH3(Y)−CH2OH
|
Similar Questions
Knowledge Check
H3C−CH2−C∣∣O−CH3NaBH4−−−−→D2O Product ?
AH3C−OD∣C∣H−CH2−CH3BH3C−OHC∣∣D−CH2−CH3CH3C−OD∣C∣D−CH2−CH3DnoneCorrect name for the given compound
CH3−CH2−C∣CH2CH3H−CH2−C∣CH3H−CH2−CH3 isA3-ethyl-5-methylheptaneB5-ethyl-3-methylheptaneC1,1-diethyl-3-methylpentaneD3-methyl-5,5-diethylpentaneChoose the incorrect statement about the following two reactions
(i) CH3−H∣C∣H−H+⋅Cl→CH3−H∣C∣H⋅+H−Cl ΔH∘=−2 kcal/mol
(ii) CH3−H∣C∣H−H+⋅Br→CH3−H∣C∣H⋅+H−Br ΔH∘=+13 kcal/molAReaction (i) would be expected to be faster than reaction (ii)BAccording to Hammound's postulate the transition state of reaction (ii) resembles the productCAccording to Hammound's postulate the transition state of reaction (i) resembles the reactantDThe C−H bond of reaction (ii) is completely broken in the transition state.
Write the structures of the products of the following reactions :
(i) CH3−CH=CH2H2O/H+−−−−−→ (ii)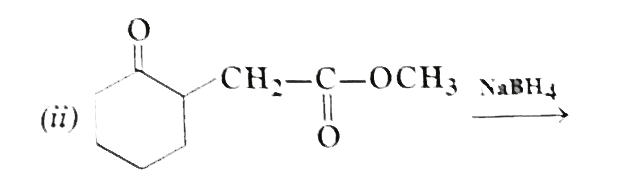
(iii) CH3−CH2−CH∣CH3−CHONaBH4−−−−→
View SolutionChoose the incorrect statement about the following two reactions
(i) CH3−H∣C∣H−H+⋅Cl→CH3−H∣C∣H⋅+H−Cl ΔH∘=−2 kcal/mol
(ii) CH3−H∣C∣H−H+⋅Br→CH3−H∣C∣H⋅+H−Br ΔH∘=+13 kcal/mol
View SolutionThe correct name of CH3CH2−C∣∣O−C∣CNH−CHO is
View SolutionChoose the correct IUPAC name for CH3−CH∣CH2−CH3−CHO is -
View SolutionIn the given reaction
CH3−CH2−C∣∣NaOH−CH3H2SO4−−−−−−−−−−→(H2O/H+),Δ(A)+(B)
A and B are:
View Solution

