2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Molecular mass of (A) is
74
88
60
102
The correct Answer is:B
ROH+CH3−Mgl→CH4+ROMgl
Number of moles of CH4= number of moles of alcohol
56022400=2.2M⇒M=[where M =molecular weight ]
Thus molecular formula of alcohol A is C5H11OH.
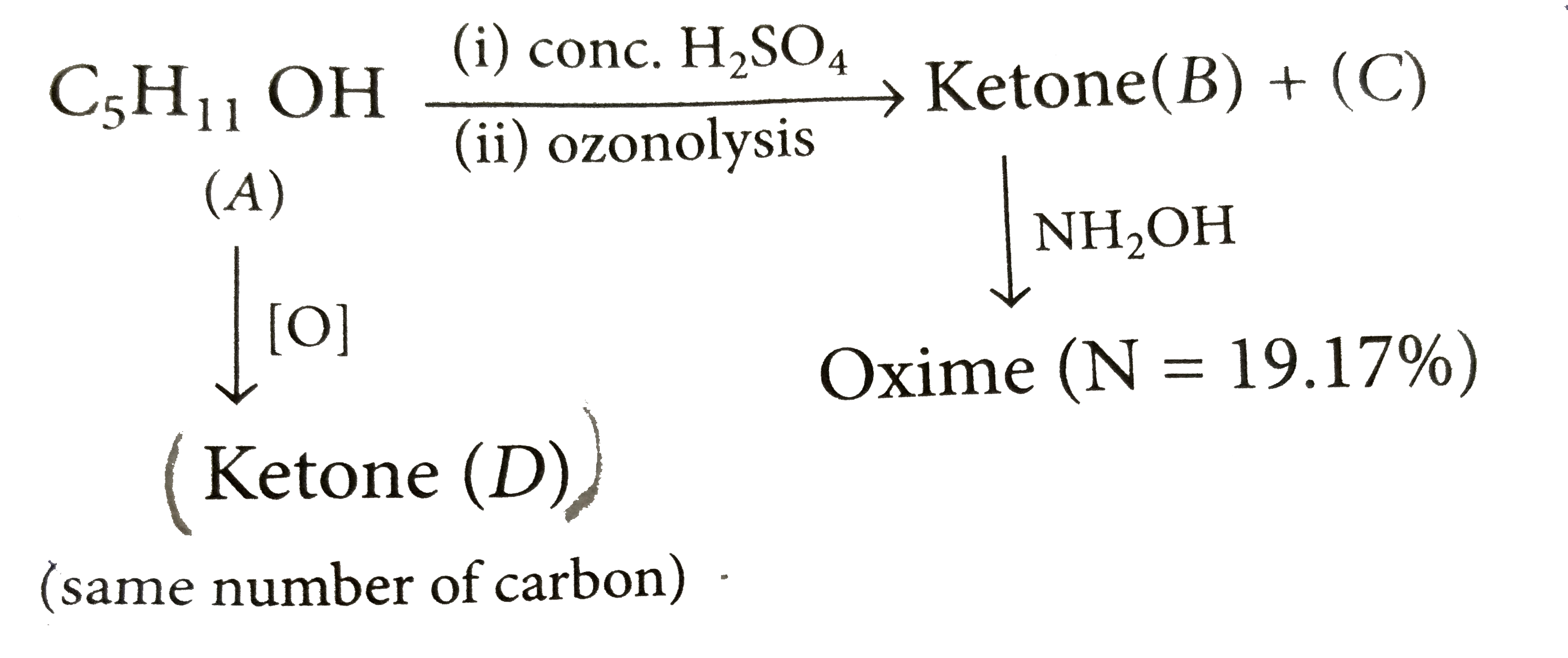
Molecular mass of
=1419.17×100=73
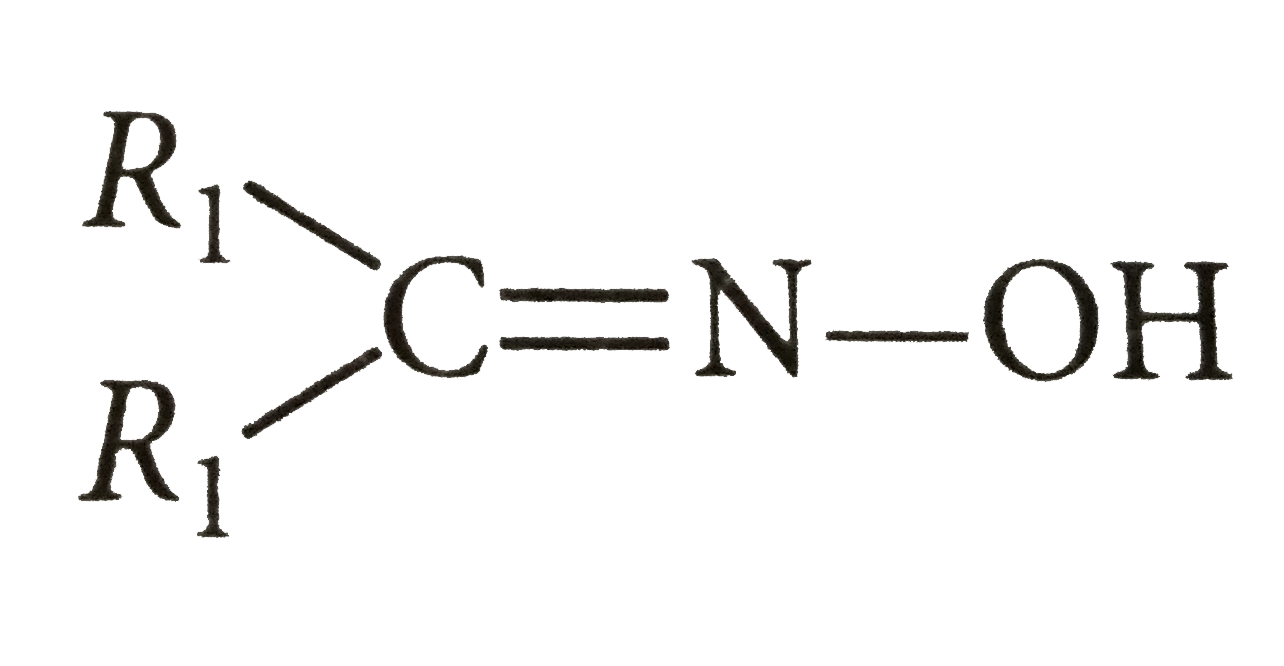
R1+R2=73−(12+14+16+1)=73−43=30
R1=R2=15CH3
Thus ketone is CH3−C∣CH3=O and hence C is CH3−CH=O.
on this basic alkene is CH3−C∣CH3=CH−CH3and
alcohol A will be CH3CH∣CH3−CH∣OH−CH3 because it is 2∘ alcohole which on oxidation gives ketone (D) having same number of C -atom.
Topper's Solved these Questions
- ALCOHOLS,PHENOLS AND ETHERSBook:NCERT FINGERTIPSChapter:ALCOHOLS,PHENOLS AND ETHERSExercise:HOTS
Explore 1 Video
- ALCOHOLS,PHENOLS AND ETHERSBook:NCERT FINGERTIPSChapter:ALCOHOLS,PHENOLS AND ETHERSExercise:NCERT Exemplar
Explore 15 Videos
- ALCOHOLS,PHENOLS AND ETHERSBook:NCERT FINGERTIPSChapter:ALCOHOLS,PHENOLS AND ETHERSExercise:Ethers
Explore 17 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NCERT FINGERTIPSChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:Assertion And Reason
Explore 15 Videos
Similar Questions
2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of (A) is
View Solution2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of ketone (D) is
View Solution
Knowledge Check
2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of (B) isACH3−CH2−O∣∣C−CH3BCH3−CH3∣C=OCCH3−C∣CH3H−O∣∣C−CH3Dnone of these2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of (B) isACH3−CH2−O∣∣C−CH3BCH3−CH3∣C=OCCH3−C∣CH3H−O∣∣C−CH3Dnone of these2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of (A) isACH3−C∣CH3H−CH2−C∣OHH−CH3BCH3−C∣CH3H−C∣OHH−CH3CCH3−CH2−C∣OHH−CH2−CH3DCH3−CH3∣C∣CH3−CH2−OH
2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of ketone (D) is
View Solution0.37g of an alcohol (A) when treated with CH3MgI liberates 112 ml of CH4 at STP. Alcohol (A) on dehydration gives alkene (B) which on ozonolysis gives acetone as one of the product along with (C). (A) when treated with benzene in acidic medium given (D) which does not give benzoc acid on oxidation. (A) on oxidation gives acid (E) having same number of C-atoms.
Q. From given data molecular formula of (A) is
View Solution0.37g of an alcohol (A) when treated with CH3MgI liberates 112 ml of CH4 at STP. Alcohol (A) on dehydration gives alkene (B) which on ozonolysis gives acetone as one of the product along with (C). (A) when treated with benzene in acidic medium given (D) which does not give benzoc acid on oxidation. (A) on oxidation gives acid (E) having same number of C-atoms.
Q. Structure of B is
View Solution0.37g of an alcohol (A) when treated with CH3MgI liberates 112 ml of CH4 at STP. Alcohol (A) on dehydration gives alkene (B) which on ozonolysis gives acetone as one of the product along with (C). (A) when treated with benzene in acidic medium given (D) which does not give benzoc acid on oxidation. (A) on oxidation gives acid (E) having same number of C-atoms.
Q. Structure of A is
View SolutionWhich alcohol on oxidation gives ketones as well as acid containing lesser number of carbon atoms than parent alcohol ?
View Solution

