0.37g of an alcohol (A) when treated with CH3MgI liberates 112 ml of CH4 at STP. Alcohol (A) on dehydration gives alkene (B) which on ozonolysis gives acetone as one of the product along with (C). (A) when treated with benzene in acidic medium given (D) which does not give benzoc acid on oxidation. (A) on oxidation gives acid (E) having same number of C-atoms.
Q. From given data molecular formula of (A) is
C5H11OH
C4H9OH
C6H13OH
C4H7OH
The correct Answer is:B
Topper's Solved these Questions
- ALKENESBook:NARAYNAChapter:ALKENESExercise:Matrix Match Type
Explore 3 Videos
- ALKENESBook:NARAYNAChapter:ALKENESExercise:Assertion reason
Explore 2 Videos
- ALKENESBook:NARAYNAChapter:ALKENESExercise:level-5 Straight Objective Questions
Explore 50 Videos
- ALKANESBook:NARAYNAChapter:ALKANESExercise:Integer Answer Type
Explore 7 Videos
- ALKYNESBook:NARAYNAChapter:ALKYNESExercise:Level-6
Explore 112 Videos
Similar Questions
An alcohol C4H9OH on dehydration gives an alkene, which on oxidation yield a acetone. The alcohol is
View Solution2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of ketone (D) is
View Solution
Knowledge Check
0.37g of an alcohol (A) when treated with CH3MgI liberates 112 ml of CH4 at STP. Alcohol (A) on dehydration gives alkene (B) which on ozonolysis gives acetone as one of the product along with (C). (A) when treated with benzene in acidic medium given (D) which does not give benzoc acid on oxidation. (A) on oxidation gives acid (E) having same number of C-atoms.
Q. Structure of B isACH3−CH2−CH=CH−CH3B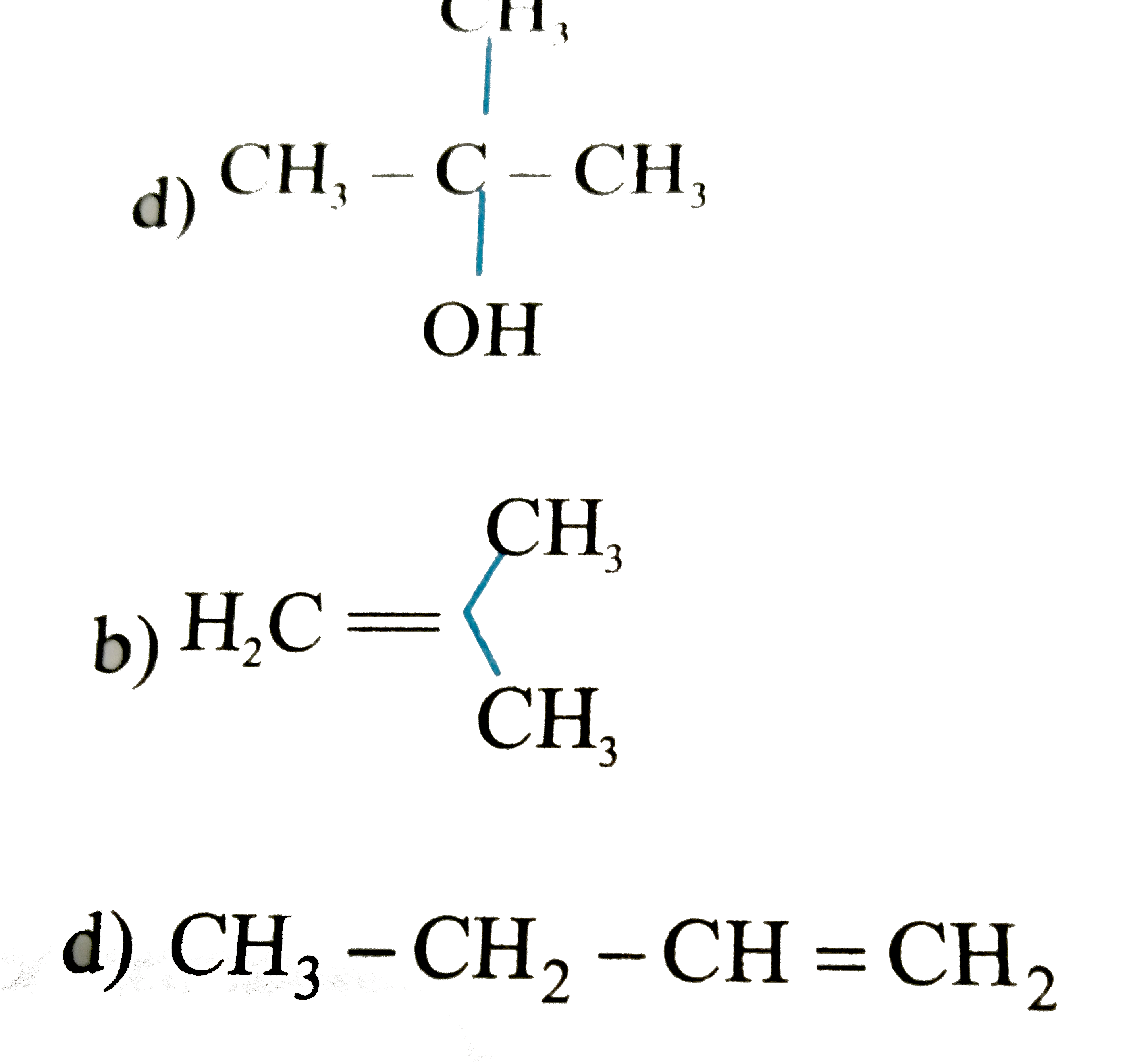 CCH3−CH3∣CH−CH2OHDCH3−CH2−CH=CH2
CCH3−CH3∣CH−CH2OHDCH3−CH2−CH=CH20.37g of an alcohol (A) when treated with CH3MgI liberates 112 ml of CH4 at STP. Alcohol (A) on dehydration gives alkene (B) which on ozonolysis gives acetone as one of the product along with (C). (A) when treated with benzene in acidic medium given (D) which does not give benzoc acid on oxidation. (A) on oxidation gives acid (E) having same number of C-atoms.
Q. Structure of A isACH3−CH2−CH−CH2−OHBCH3−CH3∣CH−CH2OHCCH3−CH2−CH∣OH−CH3DCH3−CH3∣C∣OH−CH3On oxidation of alcohol gives an acid having the same number of carbon atoms. The alcohol is,
A1∘ alcoholB2∘ alcoholC3∘ alcoholDnot necessary
2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of ketone (D) is
View Solution2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of (B) is
View Solution2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of (B) is
View Solution2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of (A) is
View Solution2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of CH4 at STP. Alcohol (A) on dehydration followed by ozonolysis gives ketone (B) along with (C). Oxime of ketone (B) contains 19.17% N. (A) on oxidation gives ketone (D) having same number of carbon atom.
Structure of (A) is
View Solution

