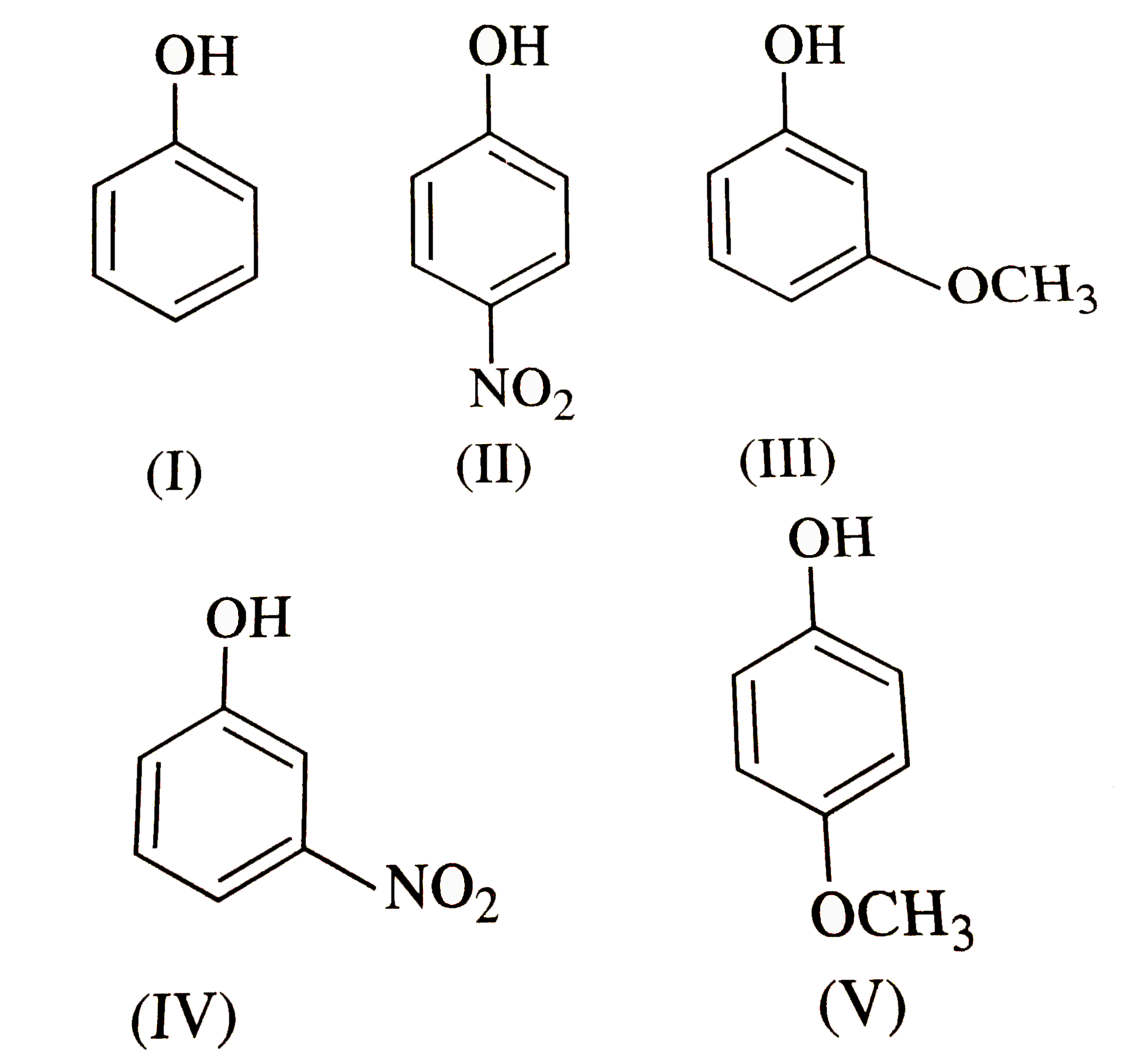
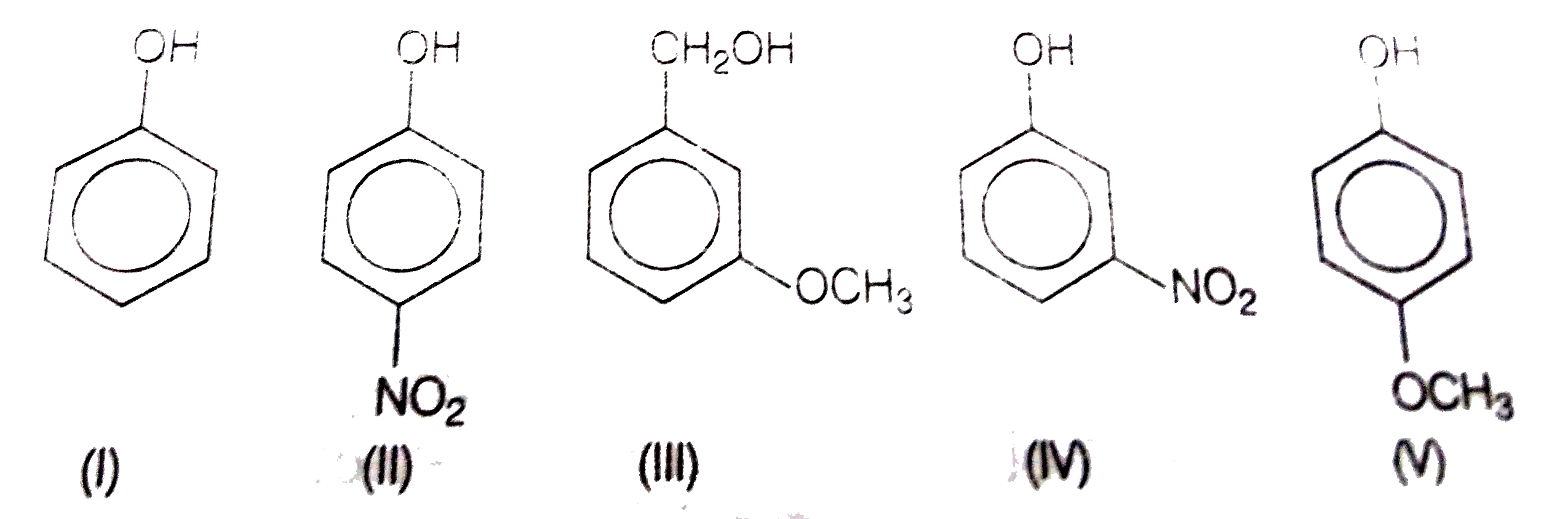
Mark the correct order of decresing acid strenght of the following compunds.
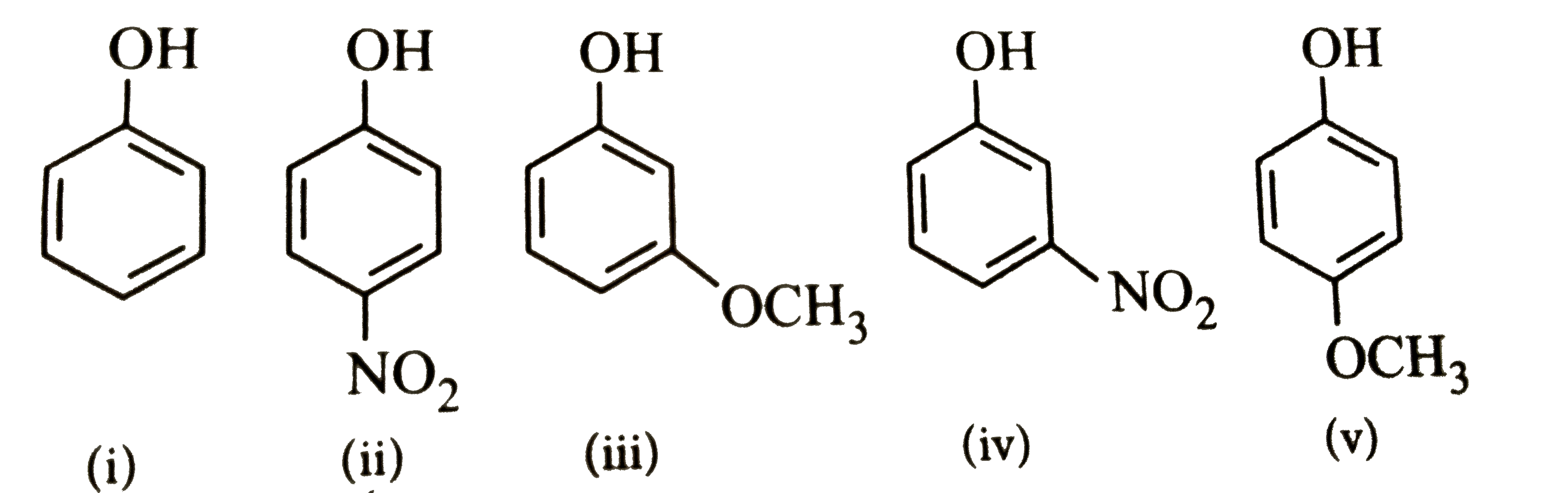
(v)>(iv)>(ii)>(i)>(iii)
(ii)>(iv)>(i)>(iii)>(v)
(iv)>(v)>(iii)>(ii)>(i)
(v)>(iv)>(iii)>(ii)>(i)
The correct Answer is:B
The electron withdrawing group (−NO2) increases the acidity of phenols and the electron donation grop (−OCH3) decreases the acidity of phenols. The effect at P-position is greater than at m-position.
Topper's Solved these Questions
- ALCOHOLS,PHENOLS AND ETHERSBook:NCERT FINGERTIPSChapter:ALCOHOLS,PHENOLS AND ETHERSExercise:ASSERTION & REASON
Explore 1 Video
- ALCOHOLS,PHENOLS AND ETHERSBook:NCERT FINGERTIPSChapter:ALCOHOLS,PHENOLS AND ETHERSExercise:Assertion And Reason
Explore 14 Videos
- ALCOHOLS,PHENOLS AND ETHERSBook:NCERT FINGERTIPSChapter:ALCOHOLS,PHENOLS AND ETHERSExercise:NCERT Exemplar
Explore 15 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NCERT FINGERTIPSChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:Assertion And Reason
Explore 15 Videos
Similar Questions
Assertion: The bond angle
 in alcohols is slighty less than the tetrahedral angle.
in alcohols is slighty less than the tetrahedral angle.
Reason : In alcohols, the oxygen of the -OH group is attached to carbon by a sigma bond formed by the overlap a sp3 hybridised orbital of carbon with sp3 hybridised orbital of oxygen .
View SolutionAssertion : Catalytic reduction of butanal gives butanol.
Reason: Aldehydes on reduction give corresponding primary alcohols.
View SolutionMonochlorination of toluene in sunlight followed by hydroysis with aq. NaOH yiedls
View SolutionAssertion : In formaldehyde all the four atoms lie in one plane.
Reason : Carbonyl carbon forms a π-bond with oxygen by overlapping of p -orbitals.
View SolutionCorrect order of acidity of I, II and III marked in following structure :
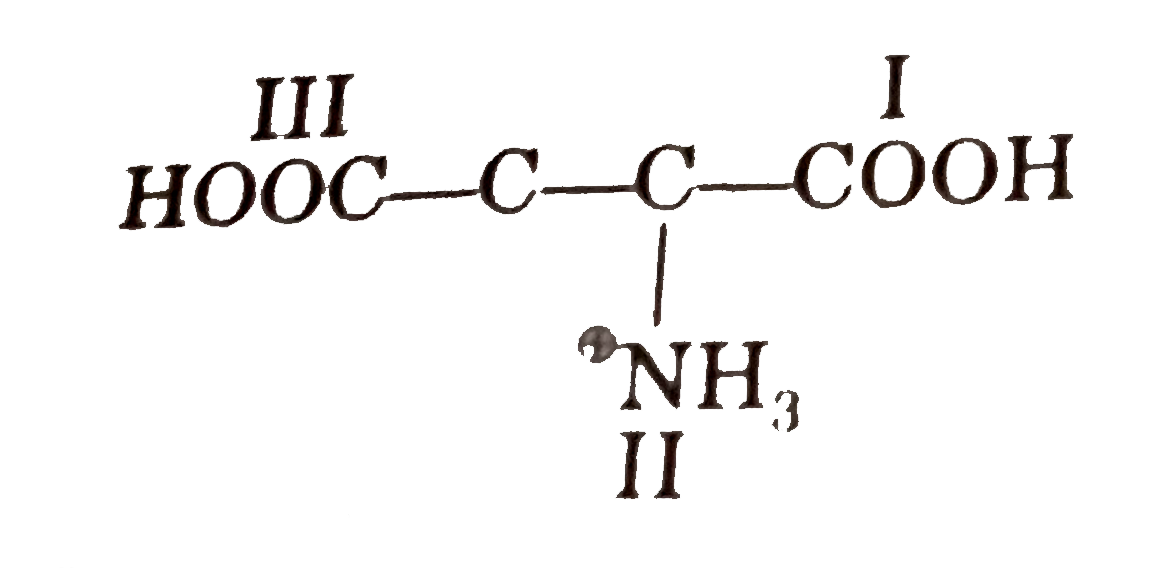
View SolutionWhich of the following is the correct order of acidic strenght ?
View SolutionArrange the following in increasing order of their acidic strength.
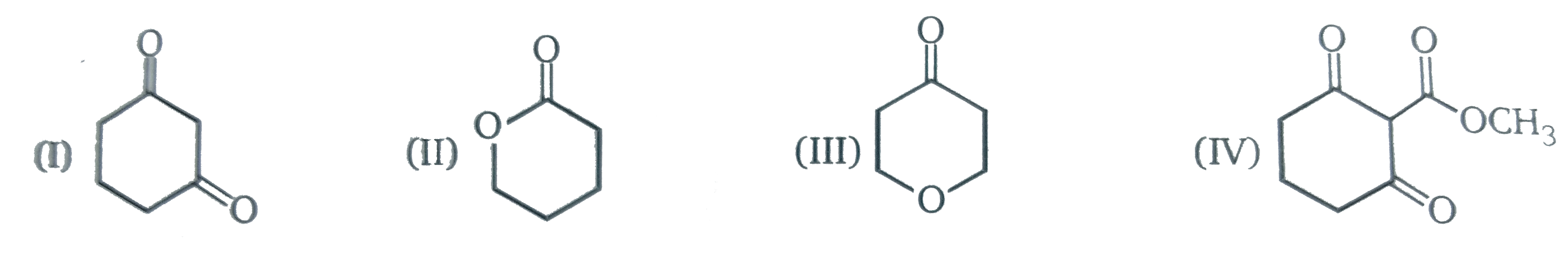
View SolutionMark the correct order of decreasing acid strength of the following compounds
View SolutionThe correct order of increasing rate of hydrolysis of the following compund is
View SolutionMark the correct order of decreasing acid strength of the following compounds.
View SolutionThe order of acidic strenght boron trihalides is:
View SolutionArrange the following acids in the decreasing order of the acidic strenght.
View SolutionWhich of the following is the CORRECT order for strength of C-X bond ?
View SolutionThe correct order of N compounds in its decresing order of oxidaton states is
View SolutionThe correct order of N-compounds in its decreasing order of oxidation states is
View SolutionThe correct order of acid strenghts of benzoic acid (X), peroxyenzoic acid (Y) and p - nitrobenzoic acid (Z) is-
View Solution

