Given below are the two reactions of H2O2. Mark the correct statement which follows.
(i) 2KMnO4+3H2SO4+5H2O2→K2SO4+2MnSO4+8H2O+5O2
(ii) 2Cr(OH)3+4NaOH+3H2O2→2Na2CrO4+8H2O
(i) Show oxidising nature of H2O2 and (ii) shows reducing nature of H2O2.
In (i) H2O2 acts as a reducing agent and in (ii) it acts as an oxidising agent.
In both (i) and (ii), H2O2 acts as an oxidising agent.
In both (i) and (ii), H2O2 acts as a reducing agent.
The correct Answer is:B
In (i) H2O2 reduces KMnO4 to MnSO4 and is oxidised to O2.
In (ii)H2O2 oxidises Cr(OH)3 to Na2CrO4 and is reduced to H2O.
Topper's Solved these Questions
- HYDROGEN Book:NCERT FINGERTIPSChapter:HYDROGEN Exercise:Heavy Water
Explore 8 Videos
- HYDROGEN Book:NCERT FINGERTIPSChapter:HYDROGEN Exercise:Higher Order Thinking Skills
Explore 9 Videos
- HYDROGEN Book:NCERT FINGERTIPSChapter:HYDROGEN Exercise:MCQs
Explore 1 Video
- HYDROCARBONS Book:NCERT FINGERTIPSChapter:HYDROCARBONS Exercise:Assertion And Reason
Explore 15 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES Book:NCERT FINGERTIPSChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES Exercise:Assertion And Reason
Explore 12 Videos
Similar Questions
What is heavy water ?
View SolutionThe varous types of hydrides and examples of each type are given below :
Hydride typeCompound(A)Electron deficient(i)LiH(B)Saline(ii)CH4(C)Electron-precise(iii)NH3(D)Interstitial(iv)B2H6(E)Electron rich(v)CrH
Choose the correct matching from the codes given below :
View SolutionChoose the correct statement about the given figures.
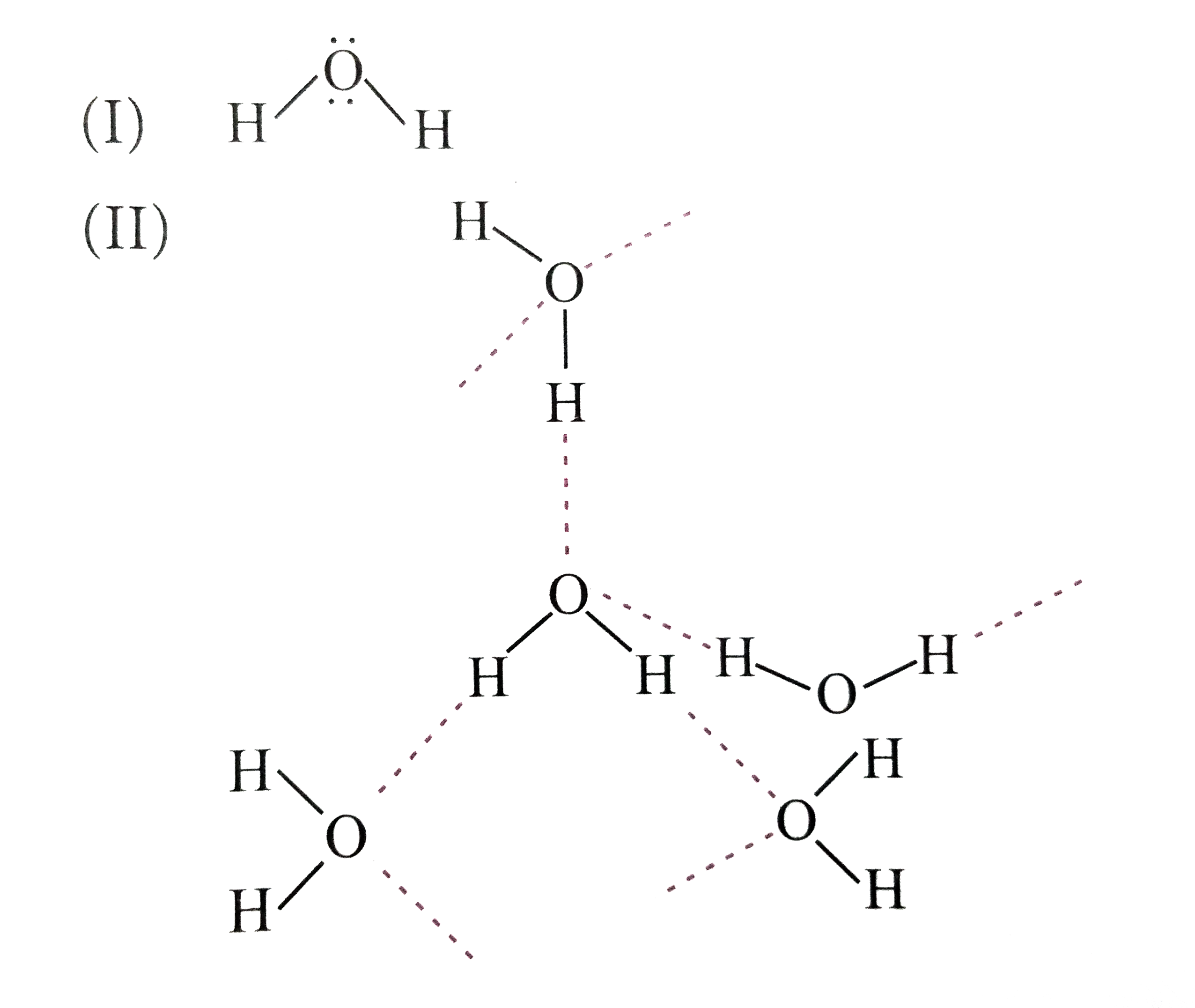
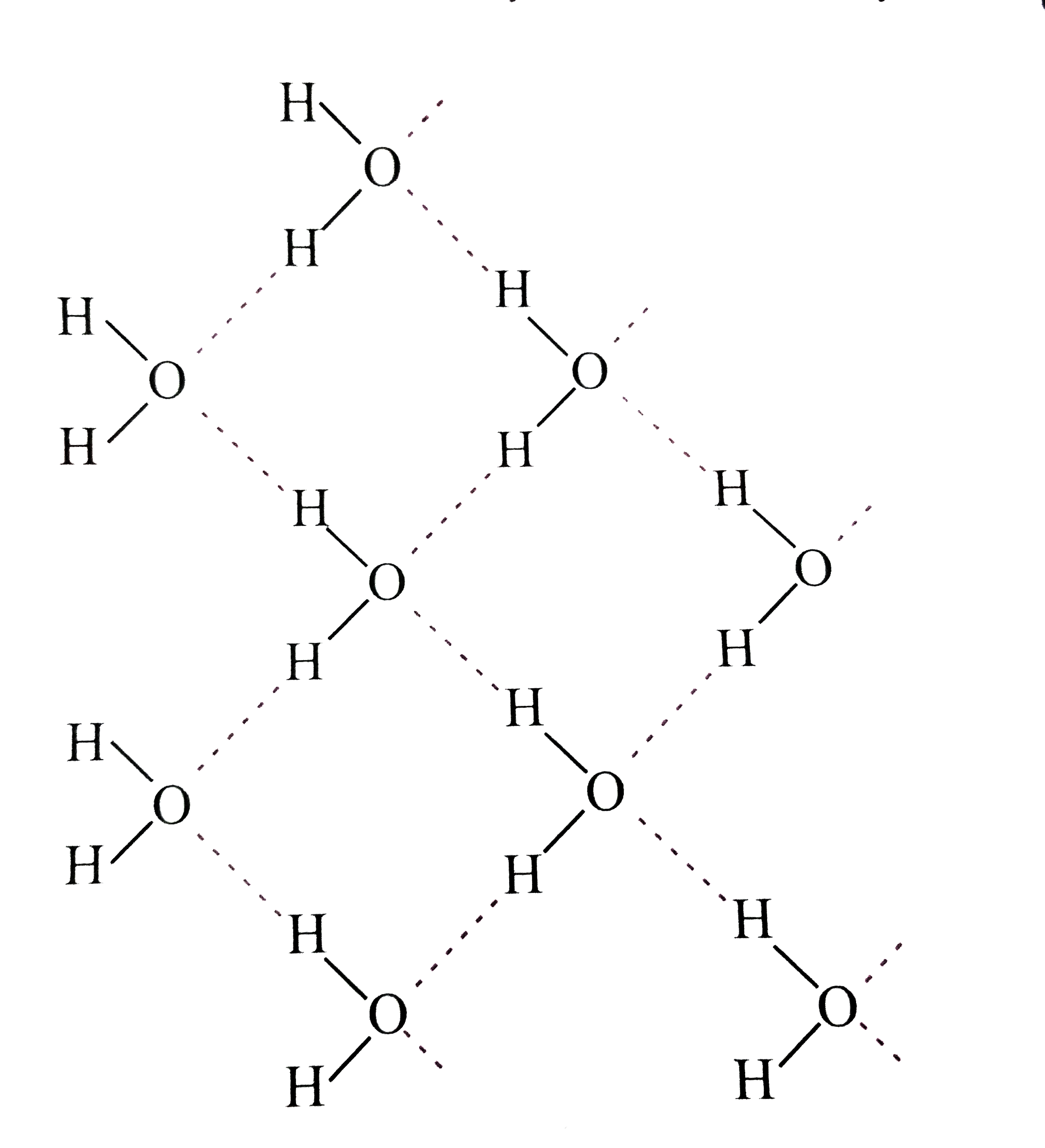
View SolutionAssertion: 2,2-Dimethylbutane does not have any tertiary carbon atom.
Reason: Tertiary carbon atom is attached to three carbon atoms.
View SolutionAssertion: Hybridisation influences the bond length and bond enthalpy in organic compound.
Reason: More the s character of hybrid orbital, shorter and stornger will be the bond.
View SolutionSelect the nature or type of redox change in the following reactions: (a) 2Cu^(+)rarrCu^(2+)+Cu^(0) (b) Cl_(2)rarrClO^(-)+Cl^(-) (c ) 2KClO_(3)overset(Delta)rarr2KCl+3O_(2) (d) (NH_(4))_(2)Cr_(2)O_(7)rarrN_(2)+Cr_(2)O_(3)+4H_(2)O (e ) 10FeSO_(4)+2KMnO_(4)+8H_(2)SO_(4)rarrK_(2)SO_(4)rarr2MnSO_(4)+5Fe_(2)(SO_(4))_(3)+K_(2)SO_(4)+8H_(2)O (f) 5H_(2)C_(2)O_(4)+2KMnO_(4)+3H_(2)SO_(4)rarrK_(2)SO_(4)+2MnSO_(4)+10CO_(2)+8H_(2)O
View Solution2MnO−4+5H2O2+6H−→2Z+5O2+8H2O. In this reaction Z is
View Solution2KMnO_(4) +3H_(2)SO_(4) rarr K_(2)SO_(4)+2MnSO_(4)+3H_(2)O+5[O] X+O rarr Y X can't be:
View SolutionIn the following reaction 2MnO4−+5H2O182+6H+→2Mn2++8H2O+5O2
The radioactive oxygen will appear in :
View Solution2KMnO_(4)+H_(2)C_(2)O_(4)toK_(2)SO_(4)+2MnSO_(4)+10CO_(2)+8H_(2)O . ltvrgt Potassium permanganate oxidises oxalic acid in acidic medium as per the above chemical reaction. Identify the coefficient of H_(2)SO_(4) in the above equatio.
View Solutionनिम्न आण्विक समीकरण को आयनिक समीकरण में बदलिए-
2KMnO4+10FeSO4+8H2SO4→5Fe2(SO4)3+8H2O+2MnSO4+K2SO4
View SolutionThe reaction, 10FeSO_(4) + 2KMnO_(4) + 8H_(2)SO_(4) to 2MnSO_(4) + 5Fe_(2)(SO_(4))_(3) + K_(2)SO_(4) + 8H_(2)O is an example of reaction of
View Solutionअभिक्रिया,
10FeSO4+2KMnO4+8H2SO4→2MnSO4+5Fe2(SO4)3+K2SO4+8H2O
उदाहरण है
View SolutionIn the following oxidation half- reaction gives -
2KMnO4+5H2C2O4+3H2SO4→K2SO4+2MnSO4+10CO2+8H2O
View SolutionFrom the given reaction 2KMnO_(4) + 3H_(2)SO_(4) + 5H_(2)O_(2) rarr K_(2)SO_(4) + 2MnSO_(4) + 8H_(2)O + 5O_(2) Find the normality of H_(2)O_(2) solution, if 20 mL of it is required to react completely with 16 mL of 0.02M KMnO_(4) solution (Molar mass of KMnO_(4) = 158 g mol^(-1) )
View Solution

