Which of the following is a true structure of H2O2 in solid phase?
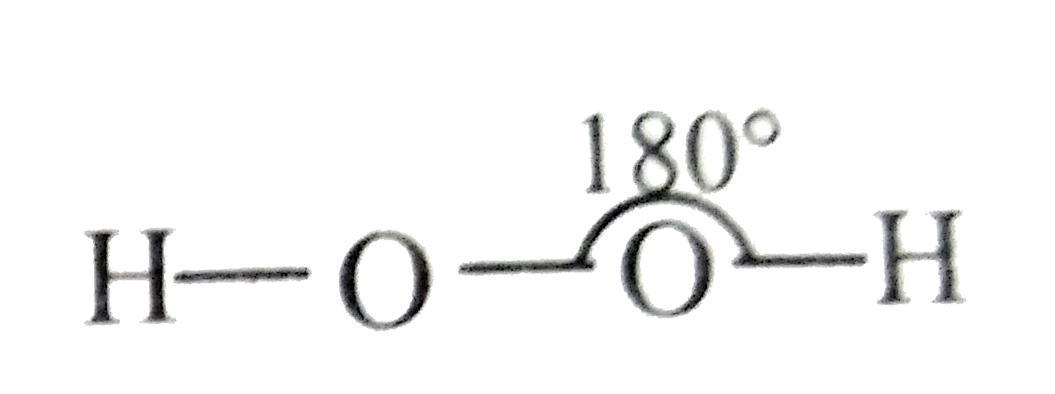
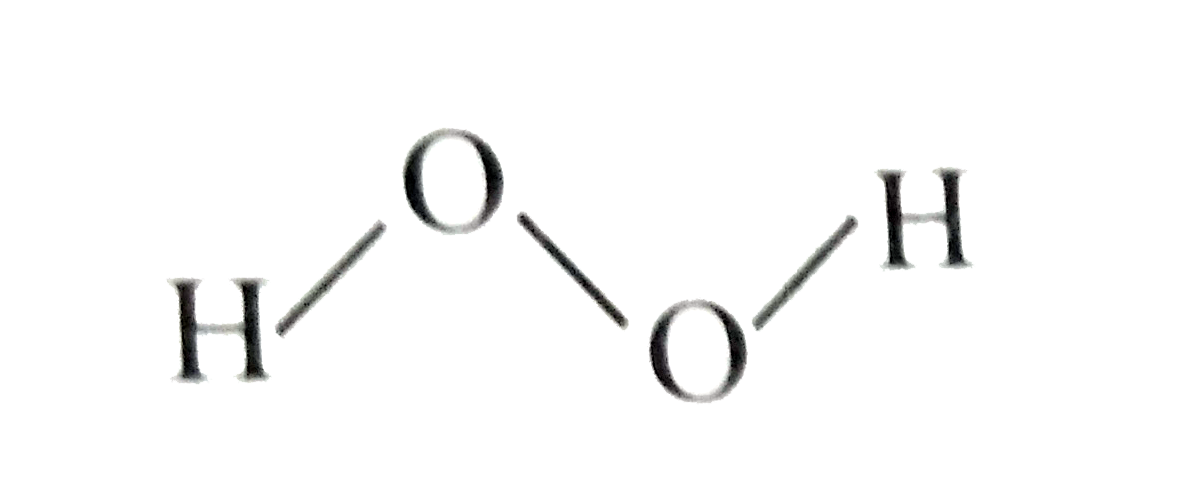
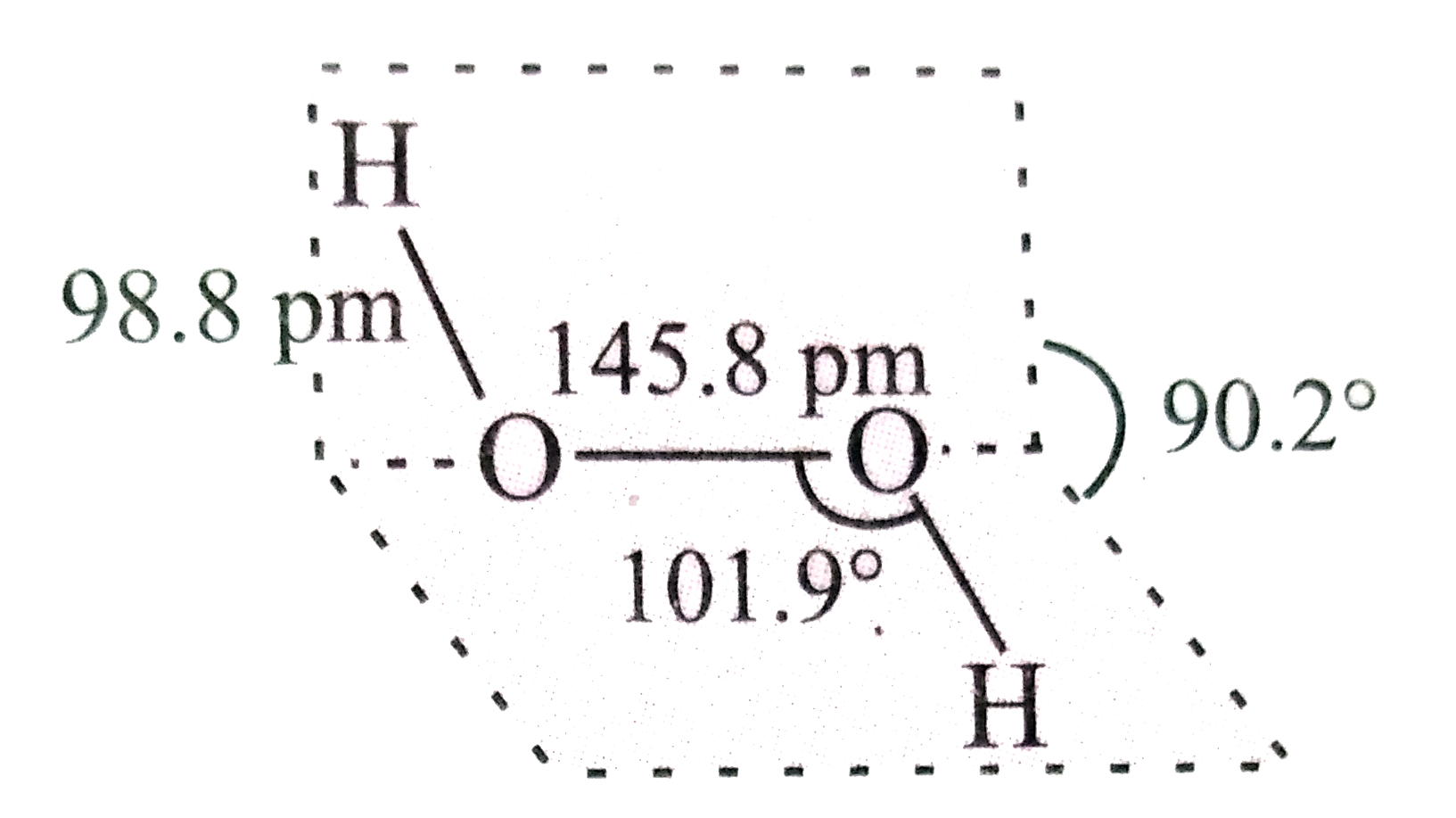
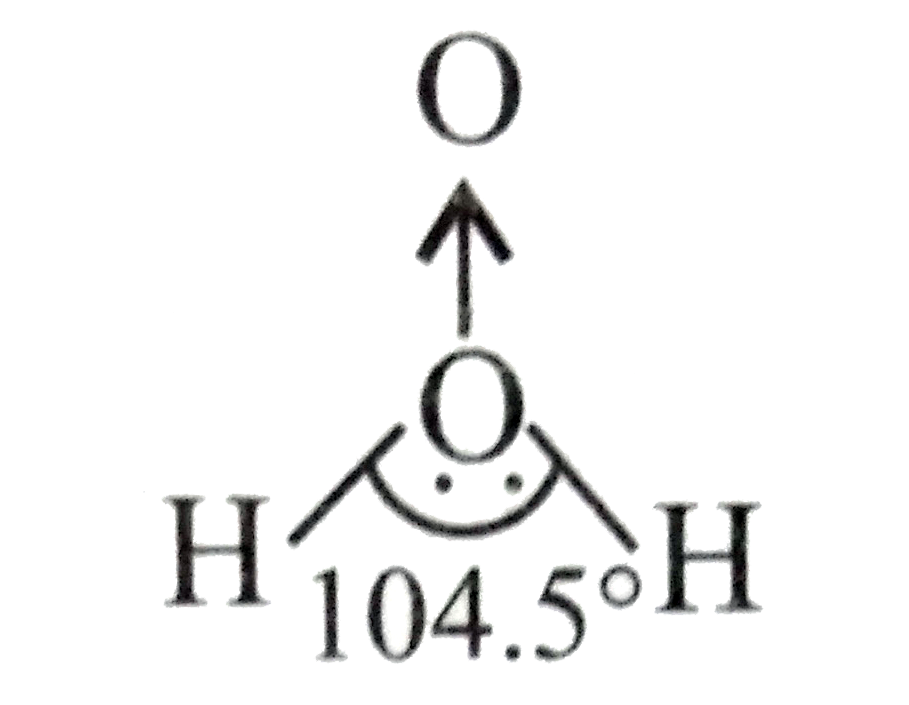
The correct Answer is:C
H2O2 has an open book structure with H-O-O bond angle equal to 101.9∘ in solid phase.
Topper's Solved these Questions
- HYDROGEN Book:NCERT FINGERTIPSChapter:HYDROGEN Exercise:Heavy Water
Explore 8 Videos
- HYDROGEN Book:NCERT FINGERTIPSChapter:HYDROGEN Exercise:Higher Order Thinking Skills
Explore 9 Videos
- HYDROGEN Book:NCERT FINGERTIPSChapter:HYDROGEN Exercise:MCQs
Explore 1 Video
- HYDROCARBONS Book:NCERT FINGERTIPSChapter:HYDROCARBONS Exercise:Assertion And Reason
Explore 15 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES Book:NCERT FINGERTIPSChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES AND TECHNIQUES Exercise:Assertion And Reason
Explore 12 Videos
Similar Questions
What is heavy water ?
View SolutionThe varous types of hydrides and examples of each type are given below :
Hydride typeCompound(A)Electron deficient(i)LiH(B)Saline(ii)CH4(C)Electron-precise(iii)NH3(D)Interstitial(iv)B2H6(E)Electron rich(v)CrH
Choose the correct matching from the codes given below :
View SolutionChoose the correct statement about the given figures.
View SolutionAssertion: 2,2-Dimethylbutane does not have any tertiary carbon atom.
Reason: Tertiary carbon atom is attached to three carbon atoms.
View SolutionAssertion: Hybridisation influences the bond length and bond enthalpy in organic compound.
Reason: More the s character of hybrid orbital, shorter and stornger will be the bond.
View SolutionThe H-OO bondangle and O-H bond lengths are 101.9^(@) and 98.8p, resectvly in solid phase instead of 94.8^(@) and 95 pm in gaseous phase instedad tat the structure of H_(2)O_(2) this indicates that the struture of H_(2)O_(2) in solid and gasous phases are different .this is due to
View SolutionChoose total number of correct statements about H2O2 :
(a) in the pure state, H2O2 is almost colourless (very pale blue)
(b) hydrogen peroxide has non-planar structure in both gas phase and solid phase
(c) 2-ethylanthraquinol react with water to give H2O2
(d) H2O2 is used in pollution control
(e) dihedral angle of H2O2 is larder in gas phase compared to that in solid phase
View Solution(i) Draw the gas phase and solid phase structure of H_(2)O_(2) . (ii) H_(2)O_(2) is a better oxidising agnet than water . Explain .
View SolutionWhich is accepted structure of H_(2)O_(2) is gas phase :
View SolutionWhich of following true structure of F_(2)O_(2) :
View Solution(i) Draw the gas phase and solid phase structrue of H_(2)O_(2) (ii) H_(2)O_(2) is a better oxidising agent than water. Explain.
View SolutionAssertion : In gaseous phase, H_(2)O and H_(2)O_(2) both have bent structures. Reason Bond angle of both H_(2)O and H_(2)O_(2) is 04.5^(@) .
View SolutionDiscuss the structure of H2O2 molecule.Explain why the shape of H2O2 molecule in the solid phase differs from that in the gas phase.
View SolutionThe dihedral angle of H_(2)O_(2) in solid phase is
View SolutionWhy the bond angle in solid phase of H_(2)O_(2) is reduced when compared to as phase of H_(2)O_(2) ?
View SolutionWhich of the following is a true structure of H2O2?
View Solution

