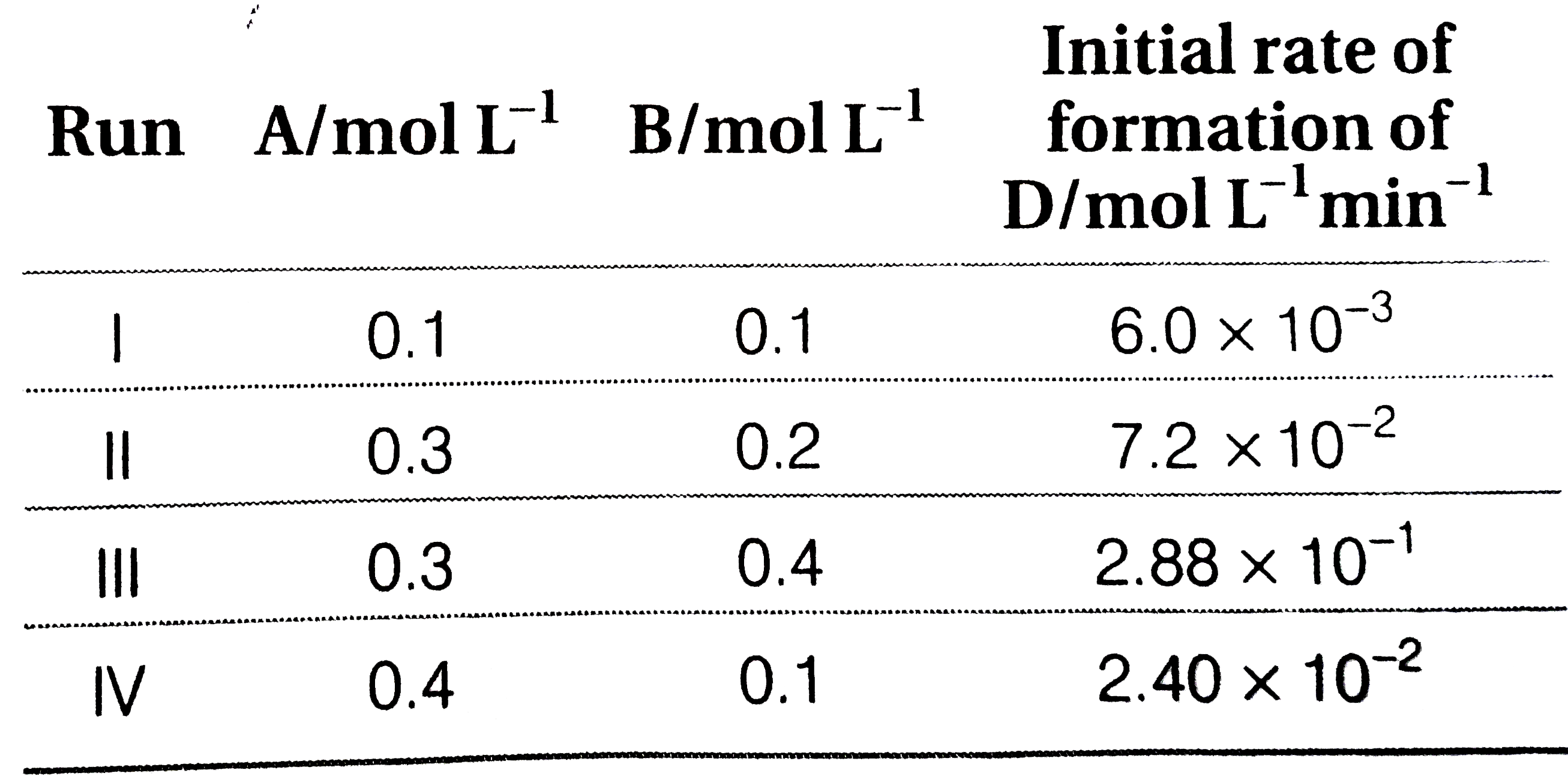
The following results have been obtained during the kinetic studies of the reaction :
2A+B→C+D
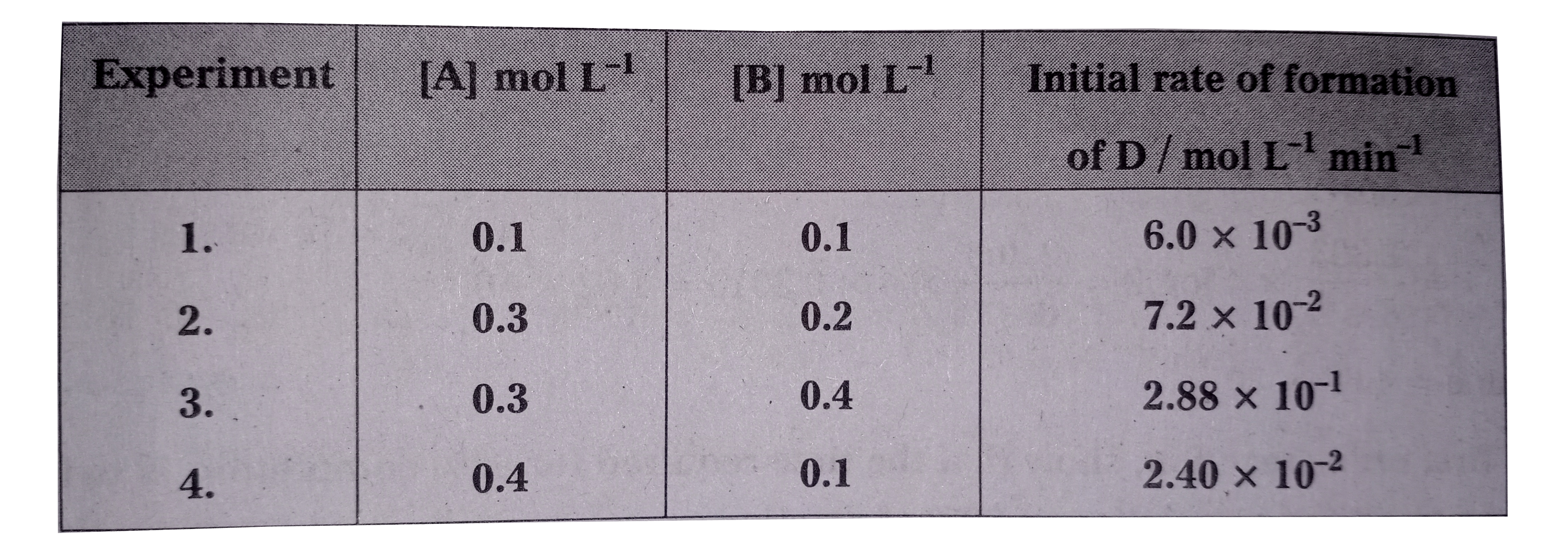
Determine the rate law and rate constant for the reaction. Solution in Telugu
Rate law may be expresswed as
Rate =k[A]x[B]y
[Rate]1=6.0×10−3=k(0.1)x(0.1)y−(i)
[Rate]2=7.2×10−2=k(0.3)x(0.2)y−(ii)
[Rate]3=2.88×10−1=k(0.3)x(0.4)y−(iii)
[Rate]4=2.40×10−2=k(0.4)x(0.1)y−(iv)
(Rate)1(Rate)4=6.0×10−32.40×10−2=k[0.1]x[0.1]yk[0.4]x[0.1]yor14=(0.1)y(0.4)x=(14)xx=1
(Rate)2(Rate)3=7.2×10−22.88×10−1=k[0.3]x[0.2]yk[0.3]x[0.4]yor14=(0.2)y(0.4)y=[12]yy=2
∴ Rate law expression is given by Rate =k[A][B]2
Rate constant k, can be determined by placing the values of A, B and rate of formation of D. By taking the values for experiment II.
Rate k[A][B]2
k=Rate[A][B]2=7.2×10−2molL−1min−1(0.3molL−1)(0.2molL−1)2=6.0mol−2L2min−1
∴k=6.0mol−2L2−1min.
Similar Questions
During the kinetic study of the reaction, 2A+Brarr C+D , following results were obtained Based on the above data which one of the following is correct?
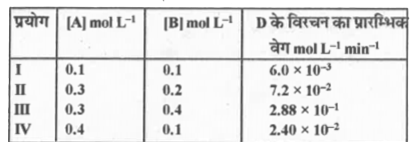
View Solution2A+B→C+Dअतः अभिक्रिया की बगलतिकी अध्यन करने पर निम्नलिखित परिणाम प्राप्त हुए। अभिक्रिया के लिए नियम तथा वेग । स्थिरांकज्ञात कीजिए।
View SolutionThe following results have been obtained during the kinetic studies of the reaction:
P+2Q→R+2S
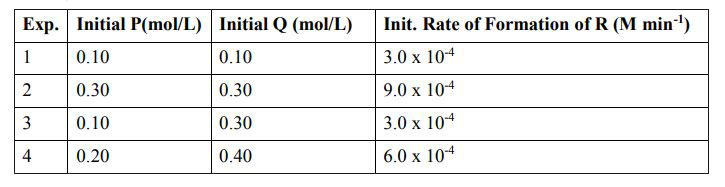
Determine the rate law expression for the reaction.
View SolutionThe following rate date were obtained at 313 K for the reaction :
2A+2B→C+D
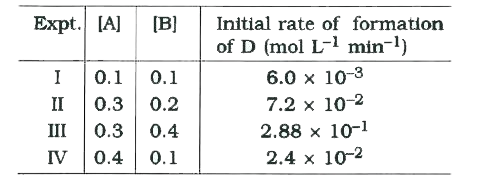
The rate law for the reaction is :
View SolutionFor the reaction 2A+B→ products, find the rate law from the following data
What is the rate constant and order of the reaction?
View SolutionFor the reaction 2N_2O_5(g) rarr 4NO_2(g)+O_2(g) the following results have been obtained. Write rate law
View SolutionDuring the kinetic study of the reaction , 2A+B→C+D, following results were obtained
Based on the above date which one of the following is correct ?
View SolutionDuring the kinetic study of the reaction, 2A+B→C+D, following results were obtained
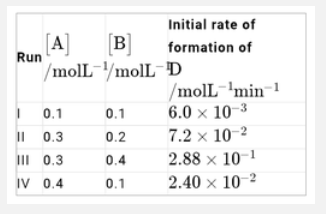
Based on the above data which one of the following is correct?
View Solution

