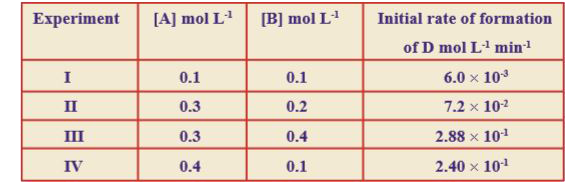
The following initial rate data were obtained at 300K for the reactions:
2A+B→C+D

Deducethe rate law.
On compairing (I) and (II), [B] remains constant. Since on making [A] twice, the reaction rate becomes four times.
(2.4×10−2/6×10−2=4), the order with respect to A is 2.
On compairing (I) and (III), [A] remains constant. Since on making [B] twice, the reaction rate becomes twice (12×10−2/6×10−2=2), the order with respect to B is 1.
∴ Rate equation is: r=k[A]2[B]
Similar Questions
The following rate data were obtained at 303 K for the following reaction:
2A+B→C+D

What is the rate law? What is the order with respect to each rectant and the overall order? What are the units of the rate constant?
View SolutionThe following rate data were obtained at 303K for the following reaction:
2A+B→C+D
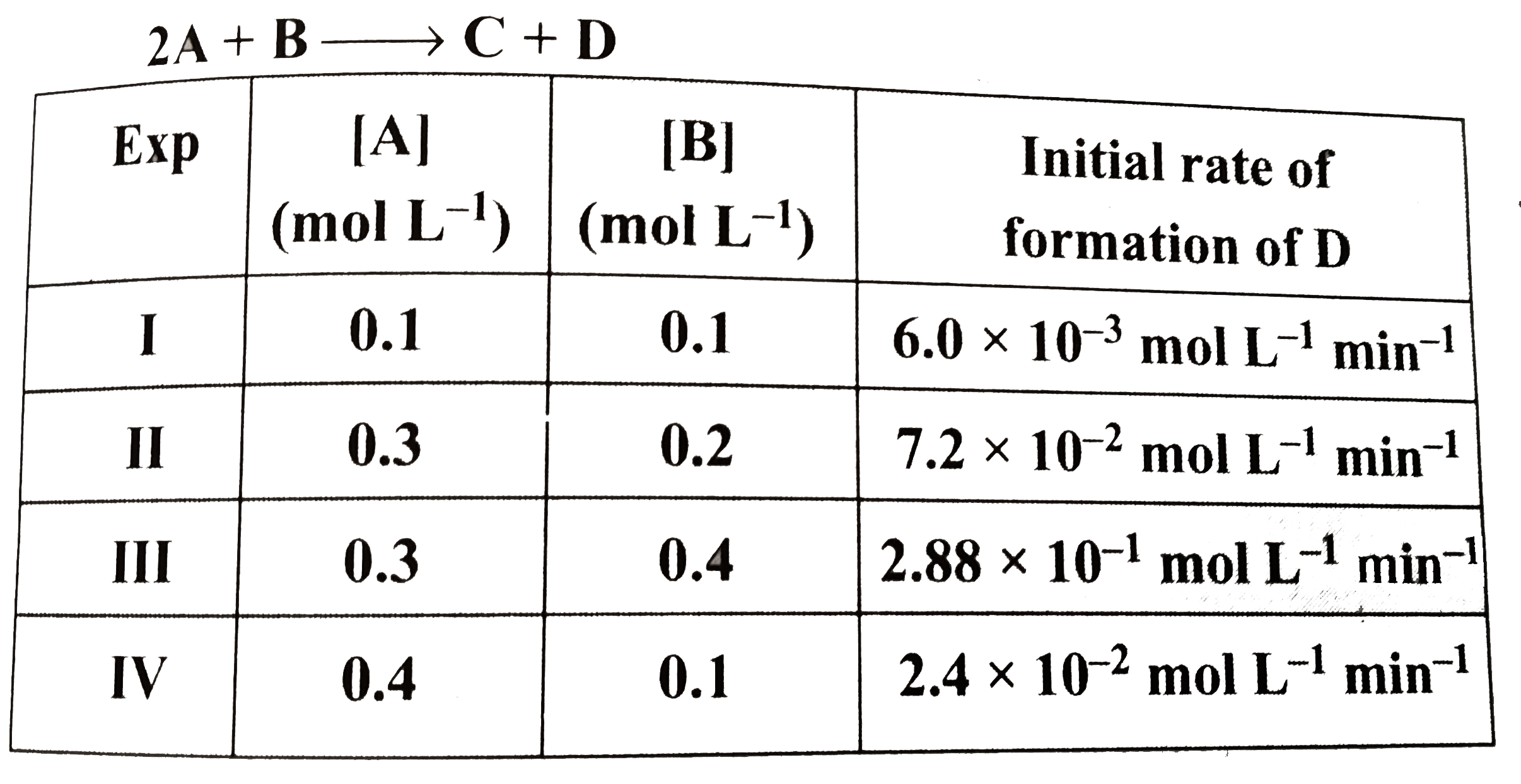
What is the rate law? What is the order with respect to each reactant and the overall order? Also calculate the rate constant and wriye its units.
View Solution
Knowledge Check
Rate law for the reaction, A+2B→C is found to be
Rate =k[A][B]
Concentration of reactant 'B' is doubled keeping the concentration of 'A' constant, the value of rate constant will be _______Athe sameBdoubledCquadrupledDhalvedForm the data given below for the reaction:A→B→ Products
the rate law expression is:ARate=k[A][B]BRate=k[A][B]2CRate=k[A]2[B]2DRate=k[A]2[B]
The following data were obtained at 300K for the reaction 2A+B→C+D

Calculate the rate of formation of D when [A] = 0.5molL−1 and [B]=0.2molL−1
View SolutionThe following initial rate data were obtianed for the reaction
2NOg+Br(g)→2NOBr(g)
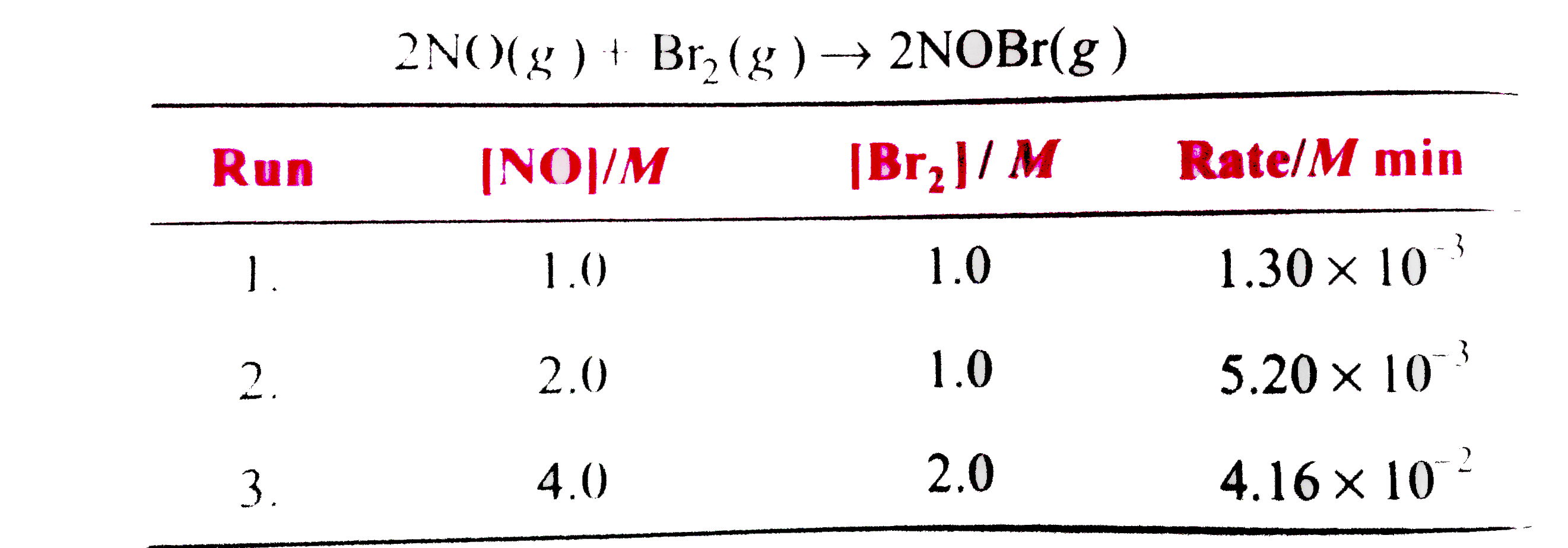
Determine the reaction rat law and the value of te rate constant.
View Solutionform the gaseous reaction
2A+B2→2AB, the following rate data were obtained at 300K.
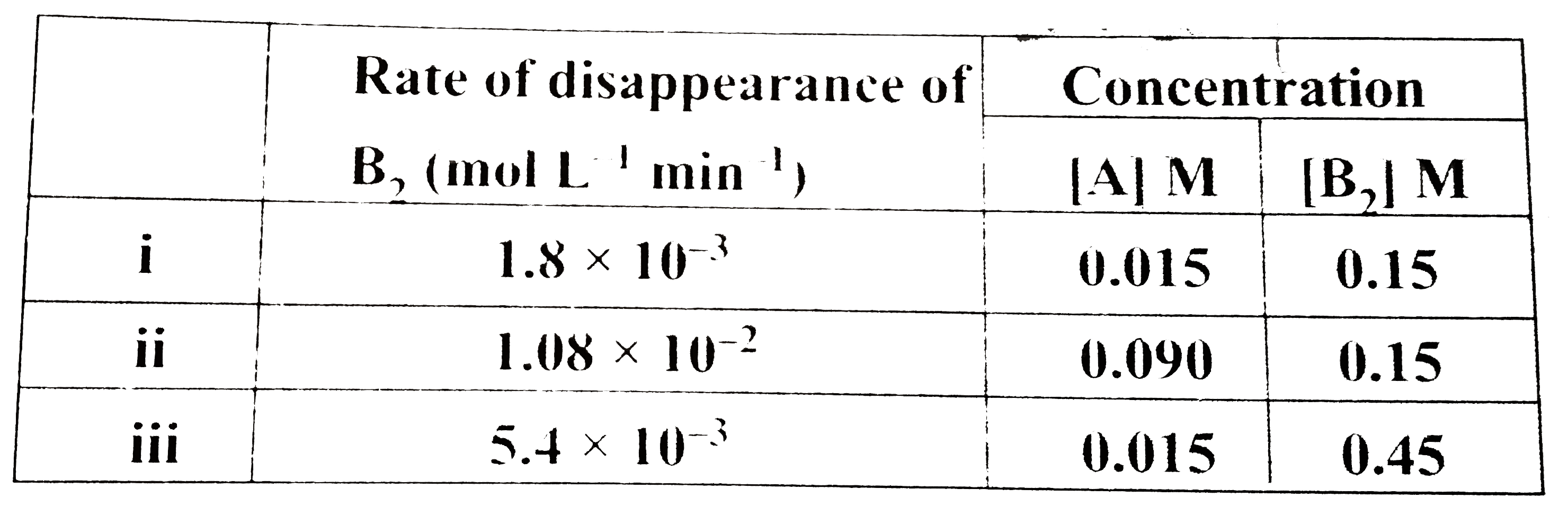
Calculate the rate constant for the reaction and the rate of formation of AB when [A] is 0.02 and [B2] is 0.04molL−1 at 300K.
View SolutionThe following results have been obtained during the kinetic studies of the reaction:
2A+B→C+D
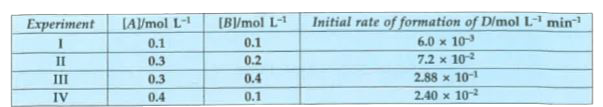
Determine the rate law and the rate constant for the reaction.
View SolutionThe following results have been obtained during the kinetics studies of the reaction :
2A+B → C + D
Determine the rate law and the rate constant for the reaction.
View SolutionFor the reaction A+B→ Products, the following initial rates were obtained at various given initial concentrations.
 ltrbgt Write rate law and find the rate constant for the above reaction.
ltrbgt Write rate law and find the rate constant for the above reaction.
View Solution

