How is ammonia manufactured by Haber's process ? Explain the reactions of ammonia with
ZnSO4(aq)Solution in Telugu
Video Solution
Text Solution
In Haber process ammonia is directly synthesised from elements (nitrogen and hydrogen). The principle involved in this is
N2(g)+3H2(g)⇔2NH3+92.4 kJ
This is a reversible exothermic reaction.
According to Le Chatelier's principle favourable conditions for the better yield of ammonia are low temperature and high pressure. But the optimum conditions are :
Temperature:720kPressure:200 atmospheresCatalyst:Finely divided iron in the presence of molybdenum (Promoter).
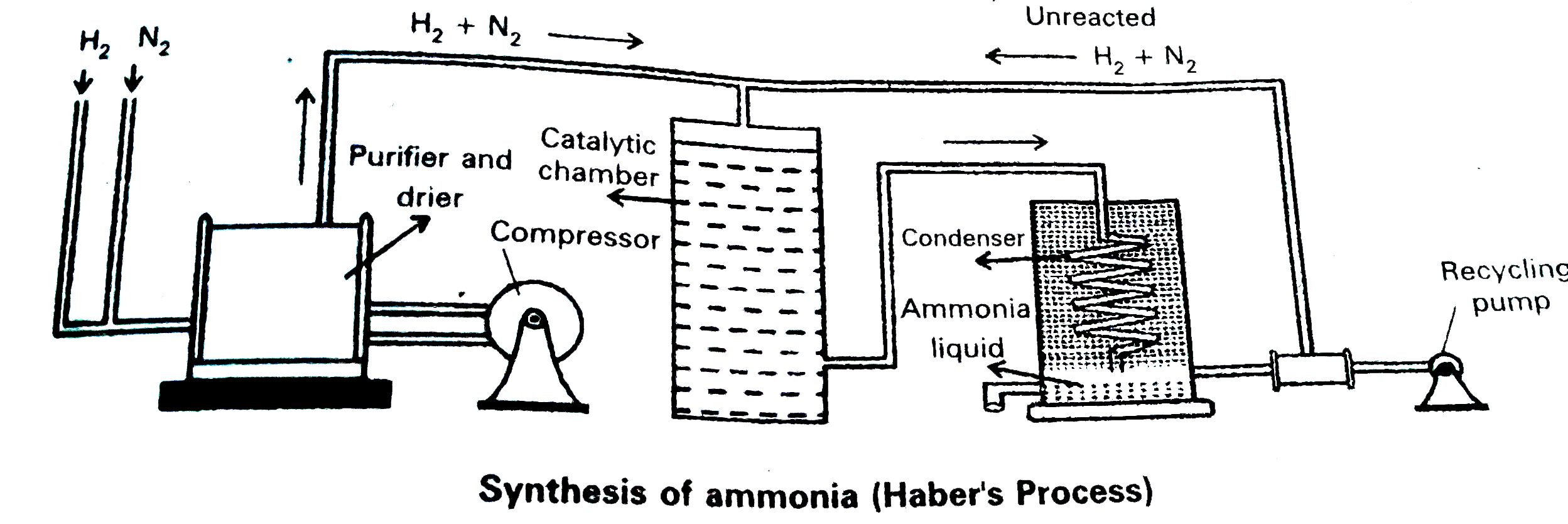
Procedure : A mixture of nitrogen and hydrogen in the volume ratio 1 : 3 is heated to 725 - 775K at a pressure of 200 atmospheres is passed over hot finely divided iron mixed with small amount of molybdenum as promotor. The gases coming out of the catalyst chamber consists of 10−20% ammonia gas are cooled and compressed, so that ammonia gas is liquified, and the uncondensed gases are sent for recirculation.
Aq. ZnSO4 reacts with ammonia aqueous solution to form white ppt of Zinc hydroxide.
NH3(aq)+H2O(l)⇔NH+4(aq)+OH+(aq)
ZnSO+4(aq)+2NH4OH(aq)→Zn(OH)2(s)+(NH4)2SO(aq)white ppt
|
Similar Questions
Explain the principles involved in the manufacture of ammonia by Haber's process.
View SolutionExplain the principles involved in the manufacture of ammonia by Haber's process.
View SolutionThe catalyst used in the manufacture of ammonia by Haber's process is
View SolutionDraw and explain the labelled flow chart of Haber's process for the manufacture of ammonia.
View SolutionWhich catalyst is used in the manufacture of ammonia by Haber's process ?
View Solution……..is used in manufacture of ammonia by Haber's process
View SolutionHow is ammonia manufactured by Haber's process?
View SolutionExplain the temperature and pressure required for manufacture of NH_3 (ammonia)by Haber's process ?
View Solution
