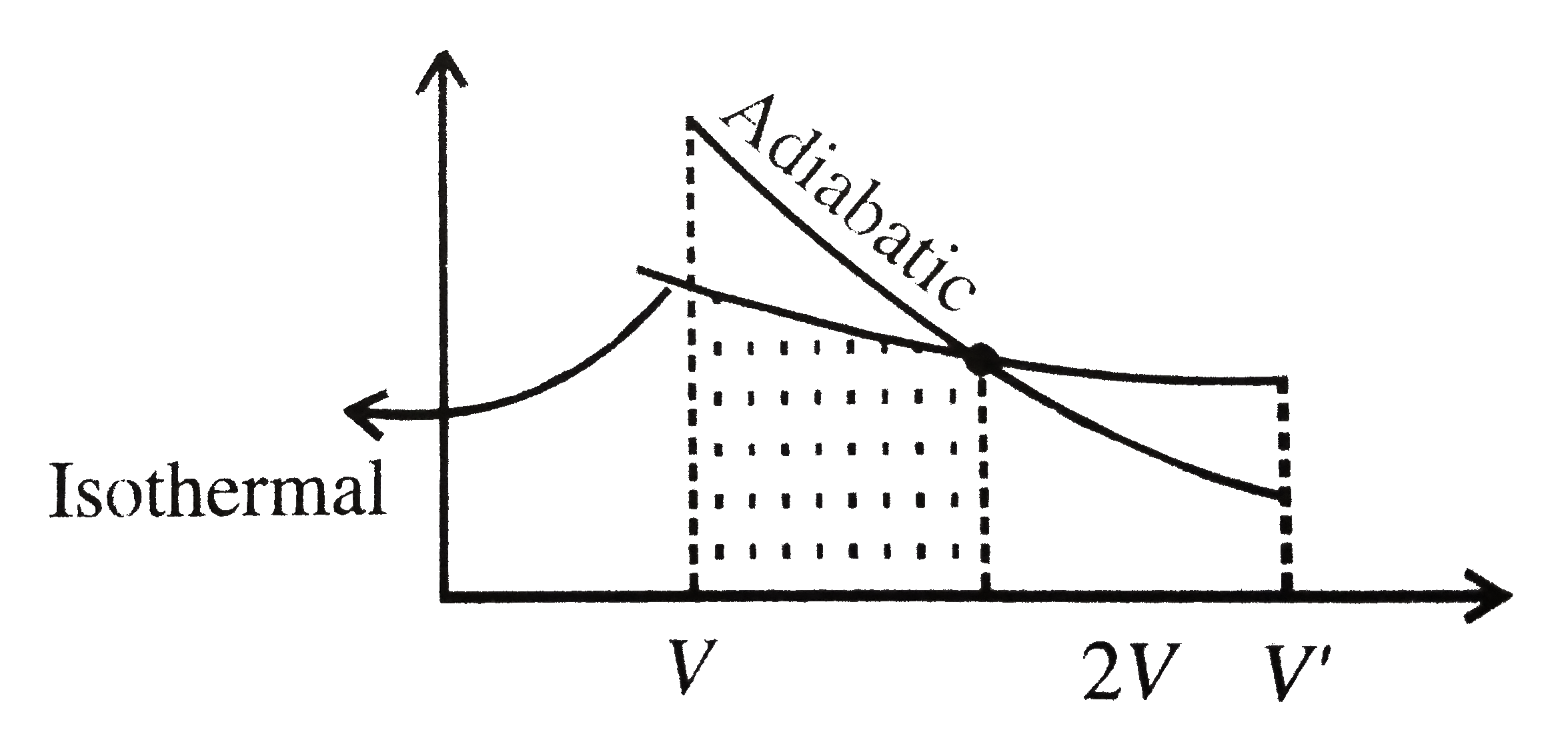
StatementI: A gas is expanded from a volume V to 2V, first through adiabatic process then through isothermal process. Work done in isothermal process is more if final state (i.e. pressure and volume ) in both the cases is same

Statement II: Work done by the gas is equal to area under p−V curve.
Statement I: is true, Statement II is true and Statement II is the correct explanation for Statement I.
Statement I: is true, Statement II is true and Statement II is NOT the correct explanation for Statement I.
Statement I is true, Statement II is false.
Statement I is false, Statement II is true.
The correct Answer is:A
When final stage in same with same initial volume, the work done in adiabatic expansion is more.
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICSChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Comprehension
Explore 56 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICSChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Interger
Explore 11 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBook:CENGAGE PHYSICSChapter:KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSExercise:Multiple Corrects
Explore 29 Videos
- KINETIC THEORY OF GASESBook:CENGAGE PHYSICSChapter:KINETIC THEORY OF GASESExercise:Compression
Explore 2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBook:CENGAGE PHYSICSChapter:LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONExercise:Multiple Correct Answer Type
Explore 9 Videos
Similar Questions
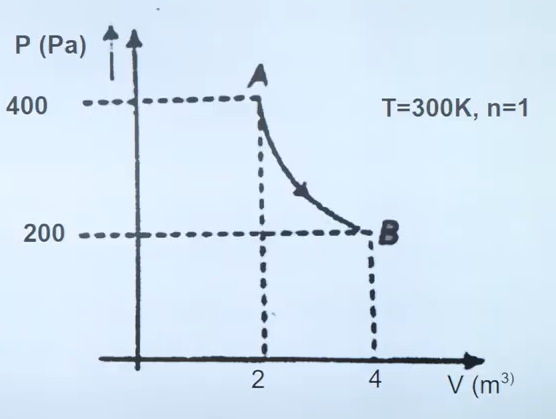
A gas is taken through an isothermal process as shown.Find the work done by the gas
View SolutionTwo samples A and B are initially kept in the same state. The sample A is expanded through an adiabatic process and the sample B through an isothermal process. The final volumes of the samples are the same .The final pressures in A and B are pA and PB respectively.
View Solution
Knowledge Check
The ratio of slopes of P−V plots for reversible adiabatic process and reversible isothermal process of an ideal gas is equal to :
AγB1−γCγ−1D1γA gas is expanded form volume V0→2V0 under three different processes as shown in the figure . Process 1 is isobaric process process 2 is isothermal and and process 3 is adiabatic .
Let ΔU1,ΔU2andΔU3 be the change in internal energy of the gs in these three processes then
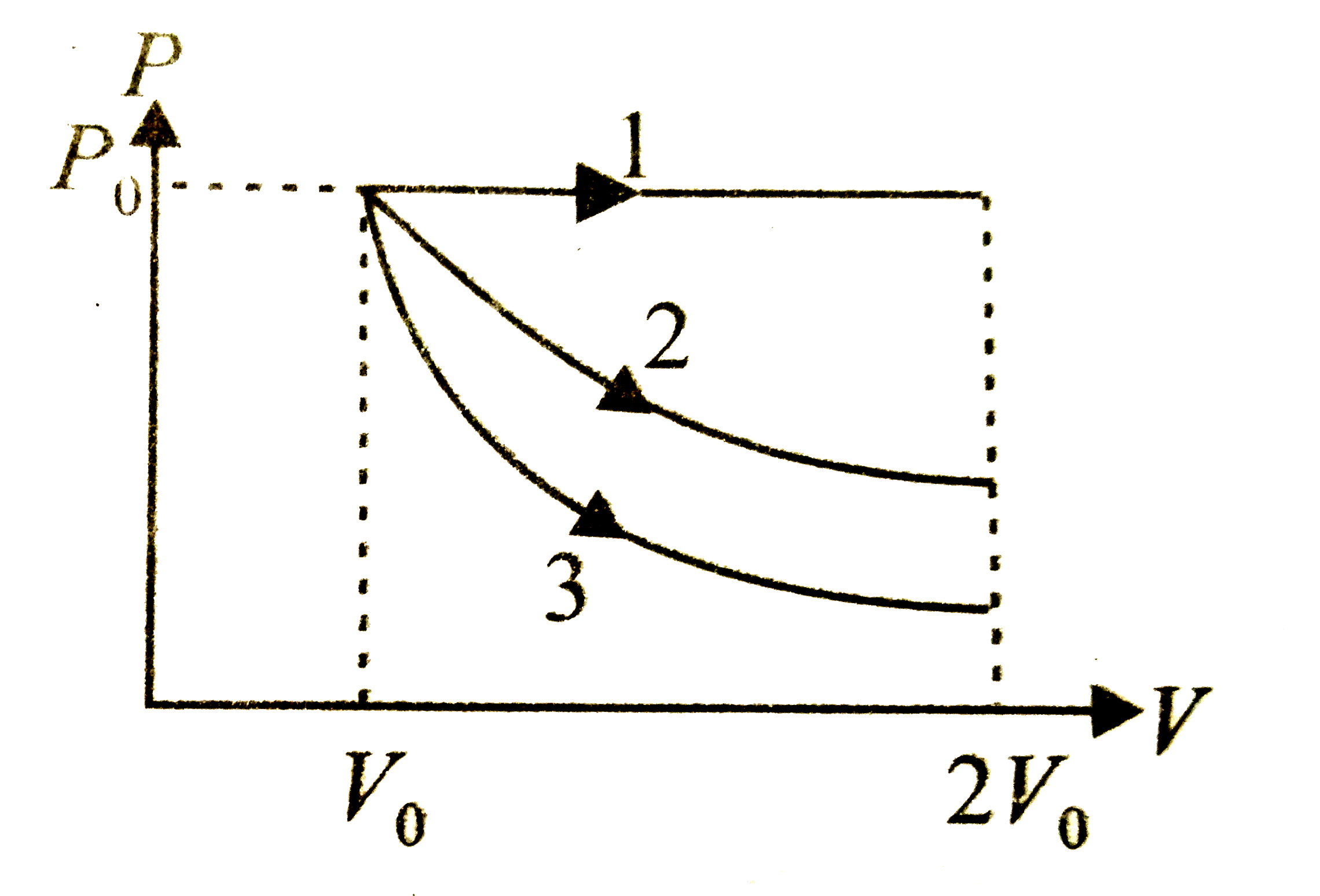 A△U1>△U2>△U3B△U1<△U3<△U2C△U2<△U3<△U3D△U2<△U3<△U1
A△U1>△U2>△U3B△U1<△U3<△U2C△U2<△U3<△U3D△U2<△U3<△U1A mono-atomic ideal gas is compressed form volume V to V/2 through various process. For which of the following processes final pressure will be maximum:
AisobaricBisothermalCadiabaticDPV2= constant
A gas is expanded from volume V1 to V2 through three different process:
a. Reversible adiabatic
b. Reversible isothermal
c. Irreversible adiabatic (against a constant external pressure Pex)
The correct option is
View SolutionA gas may expand either abiabatically or isothermally .A number of P-V curves are drawn for the two processes over different ranges of pressure and volume . It will be found that :
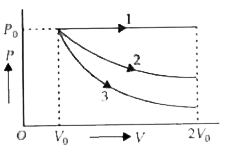
View SolutionA gas is expanded from volume V0 to 2V0 under three different processes, as shown in the figure. Process 1 is isobaric process, process 2 is isothermal and process 3 is adiabatic. Let ΔU1,ΔU2 and ΔU3 be the change in internal energy of the gas in these three processes. Then
View SolutionAn ideal gas expands from an intial volume V into vacuume under isothermal conditions. For this process,
View SolutionA gas may expand either abiabatically or isothermally .A number of P-V curves are drawn for the two processes over different ranges of pressure and volume . It will be found that :
View Solution
