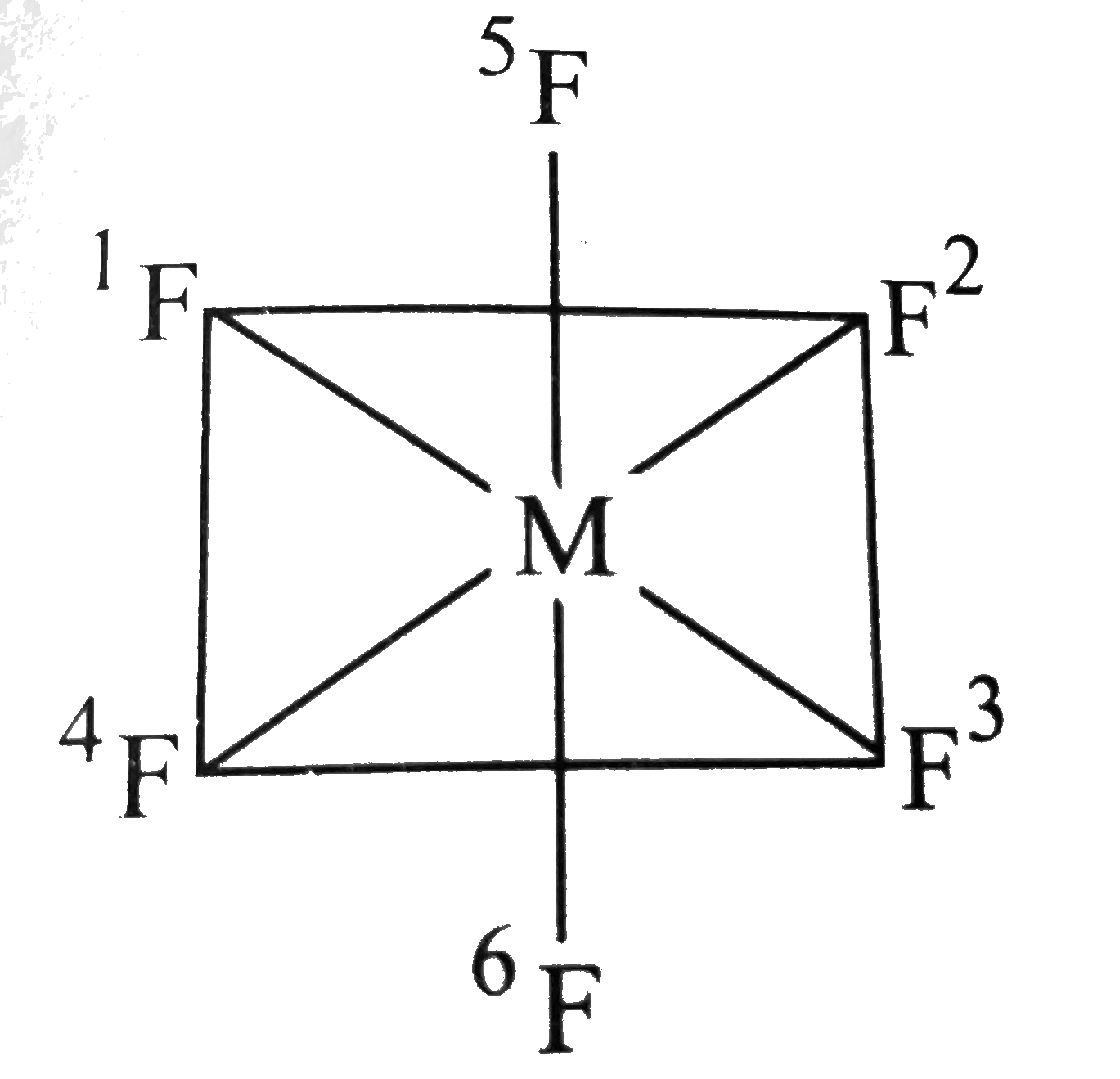
In a regular octahedral molecule SF6 the number of F−M−F bonds at 180∘ is .
A
2
B
3
C
4
D
6
Video Solution
Text Solution
The correct Answer is:B
|
Similar Questions
Knowledge Check
In a regular octahedral molecule MX6 the number of X−M−X bonds at 180∘ is
AthreeBtwoCsixDfourIn a regular octahedral molecule MX6 the number of X−M−X bonds at 180∘ is
A3B2C6D4In a regular octahedral molecule, MXg the number X-M-X bonds at 180∘ is:
ATwoBSixCFourDThree
In a regular octahedral molecule MX6 the number of X−M−X bonds at 180∘ is
View SolutionIn a regular trigonal bipyramidal MX5, the number of X - M - X bonds at 180∘ is
View SolutionWhich of the following statements is/are correct for H2C=SF4?
(I) Double bond lies in equatorial position.
(II) It is a non-planar and polar molecule.
(III) Maximum number of atoms in a plane is 6.
View SolutionThe number of bonding pairs of electron of SF4 is
View SolutionIn water molecule, the two O - H bonds are oriented at an angle of 104.5∘ . In BF3, the three B - F bonds are oriented at an angle of 120∘ . In BeF2, the two Be - F bonds are oriented at an angle of 180∘. Which of the following will have highest dipole moment ?
View Solution