Which of the following pairs of elements would you expect to have lower first ionisation energy?
a.Cl or F
b.Cl or S
c.K or Ar
d.Kr or Xe
Video Solution
Text Solution
a. Cl b.S c. K d. Xe
|
Similar Questions
Knowledge Check
Which of the following compounds would you expect to have a dipole moment ?
A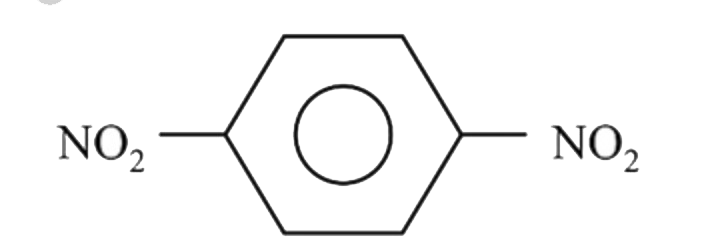 B
B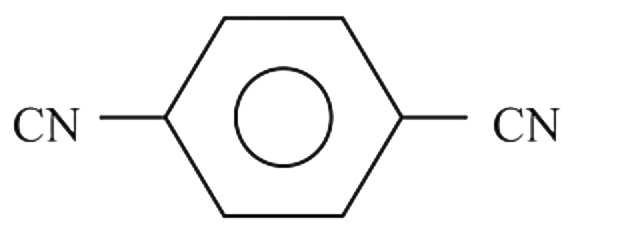 C
C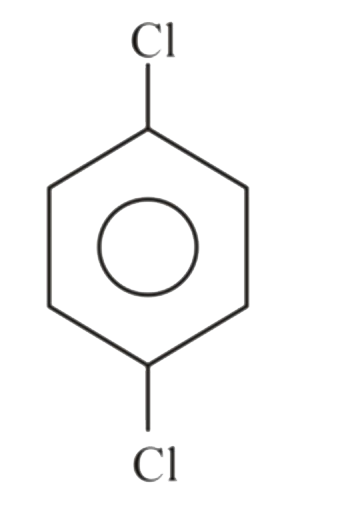 D
D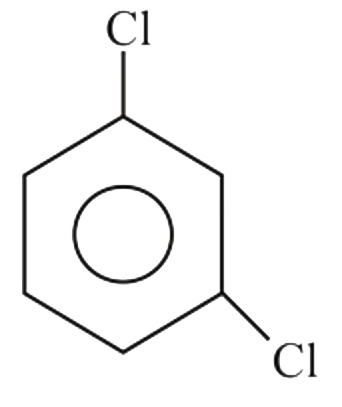
Which of the following animals would you expect to have the most efficient kidneys?
AHumansBSmall desert mammalsCMarine fishDFresh water fishWhich of the element is expected to have lowest first ionisation enthalpy
ASrBAlCXeDS.
Which of the following pairs of elements would have a more negative electron gain enthalpy?
a. O or F, b.F or Cl
View SolutionWhich of the following pairs of elements would have a negative electron gain enthalpy ?
(i) O or F (ii) F or Cl
View SolutionWhich of the following pairs of species would you expect to have largest difference in spin only magnetic moment ?
View SolutionWhich of the following elements will have the lowest first ionisation energy?
View SolutionWhich one of the following order is correct for the first ionisation energies of the elements?
View Solution
