Arrange the alkali metals in increasing order of their density.
Video Solution
Text Solution
Li<K<Na<Rb<Cs
|
Topper's Solved these Questions
- APPENDIX - INORGANIC VOLUME 1Book:CENGAGE CHEMISTRYChapter:APPENDIX - INORGANIC VOLUME 1Exercise:chapter-2 Multiple correct answer
Explore 2 Videos
- APPENDIX - INORGANIC VOLUME 1Book:CENGAGE CHEMISTRYChapter:APPENDIX - INORGANIC VOLUME 1Exercise:chapter-2 Single correct answer
Explore 3 Videos
- APPENDIX - INORGANIC VOLUME 1Book:CENGAGE CHEMISTRYChapter:APPENDIX - INORGANIC VOLUME 1Exercise:Exercise very short
Explore 70 Videos
- ALKYNESBook:CENGAGE CHEMISTRYChapter:ALKYNESExercise:Exercises (Archives - Analytical and Desriptive Type)
Explore 4 Videos
- ATOMIC STRUCTUREBook:CENGAGE CHEMISTRYChapter:ATOMIC STRUCTUREExercise:Concept Applicationexercise(4.3)
Explore 19 Videos
Similar Questions
Knowledge Check
Arrange the following metals in the increasing order of their densities. K,Sc, Ti and Ca
AK<Ca<Sc<TiBTi<Sc<Ca<KCSc<Ti<K<CaDSc<Ti<Ca<KArrange the following in increasing order of their acidic strength.
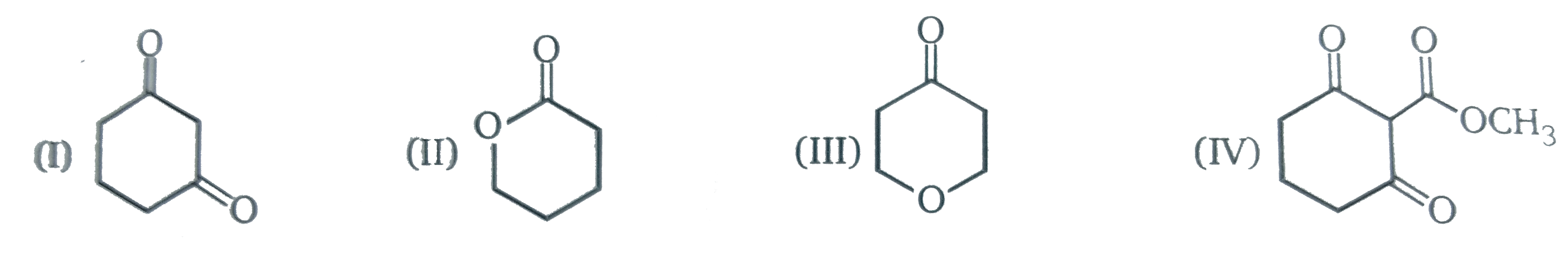 AIII<I<IV<IIBII<I<IV<IIICI<III<IV<IIDII<III<I<IV
AIII<I<IV<IIBII<I<IV<IIICI<III<IV<IIDII<III<I<IVIncreasing order of density is
ALi<K<Na<Rb<CsBLi<Na<K<Rb<CsCLi<Na<K<Cs<RbDK<Li<Na<RB<Cs
Arrange the following pkb in increasing order
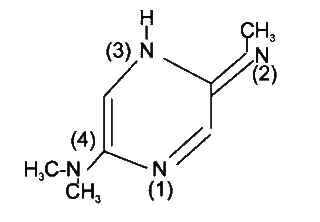
View SolutionName alkali metals in increasing order of atomic number.
View Solution
Arrange the following compounds in increasing order of their densities:
View SolutionArrange the following alkali metal ion in decreasing order of their mobility : Li+,Na+,K+,Rb+,Cs+
View SolutionArrange alkali metal carbonate in increasing order of solubility.
View Solution
