Suggest reasons why the BF bond length in BF3(130 pm) and BF_(4)^(ө)(143 pm) differ.
Text Solution
In BF3,B is sp2 hybridised and has only six electrons in the valence shell and behaves as an electron-deficient compound. To reduce its electron deficiency, B accept a pair of electron from filled 2p orbital present on it. This is known as pπ−pπ back bonding which happens due to compatibility in the size and energy of 2porbital on F and 2p orbital on B atom in BF3.
Due to back-bonding in BF3 B−F bond acquires some double bond character resulting in shortening of the bond.
However, in [BF4](ө),B is sp3 hybridised and hence has eight electrons in its valence shell. Since there is an empty p-orbital available in B in [BF4](ө) ion, back-bonding is not possible and B−F is purely single bond. Since double bonds are shorter than single bond, the B−F bond length in BF3 is shorter than in [BF4](ө).
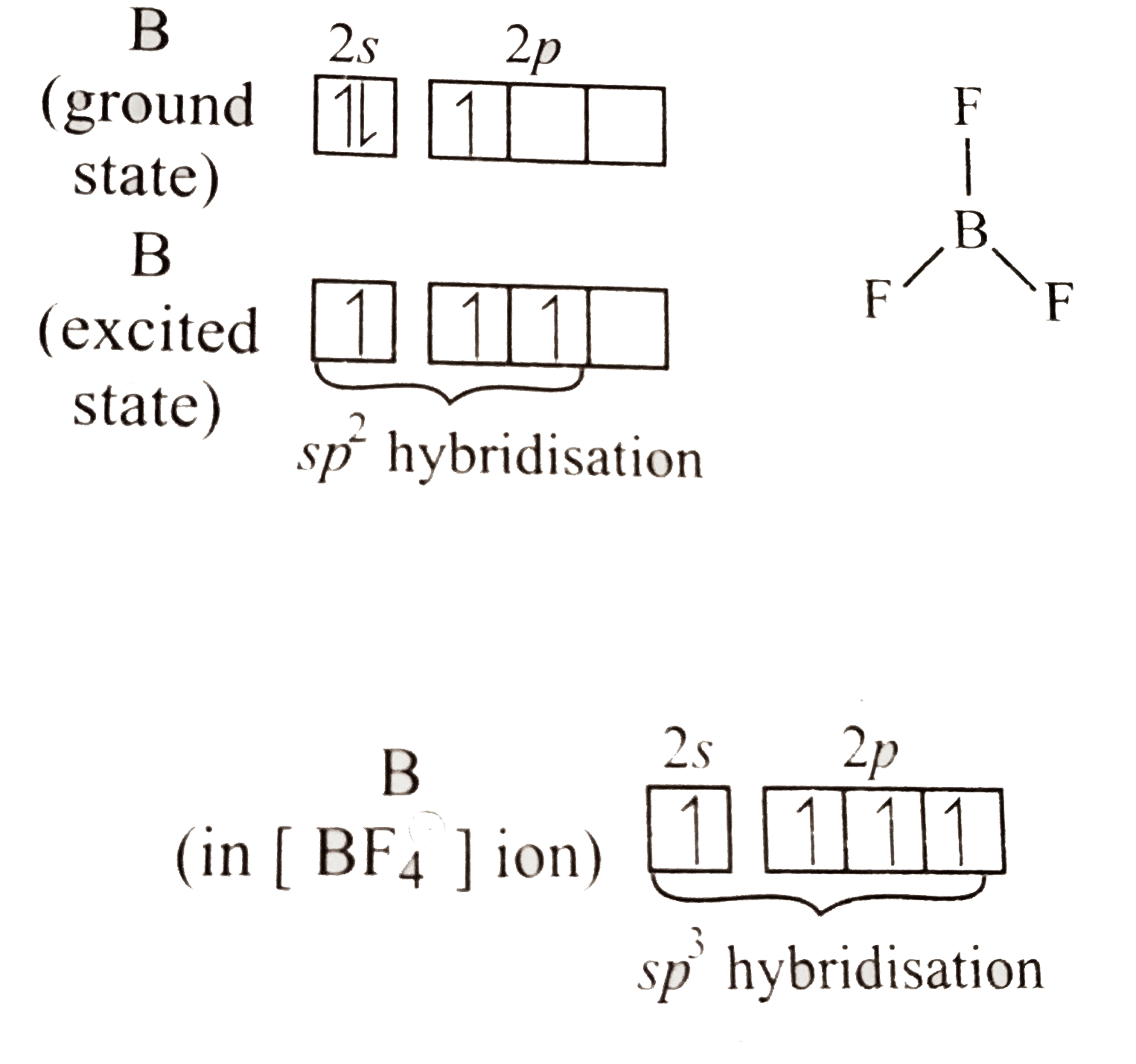

|
Similar Questions
Knowledge Check
Back bonding in BF3 does not afect
Aplanarity, lewis acidic strength and bond angleBbond length, hybridisation and bond strengthCbond angle, planarity, geometryDLewis acidity, bond length, bond order (B-F)Back bonding in BF3 does not afect
Aplanarity, lewis acidic strength and bond angleBbond length, hybridisation and bond strengthCbond angle, planarity, geometryDLewis acidity, bond length, bond order (B-F)
Suggest reasons why the B–F bond lengths in BF3 (130 pm) and BF–4(143 pm) differ.
View Solution(a) Explain why BF3 exists whereas BH3 does not.
(b) Compare the B-F bond length in BF3 and BF−4
View Solution(a) Explain why BF3 exists whereas BH3 does not.
(b) Compare the B-F bond length in BF3 and BF−4
View Solution(a) Explain why BF3 exists whereas BH3 does not.
(b) Compare the B-F bond length in BF3 and BF−4
View SolutionB — F bond length in BF3 and [BF4]−are respectively -
View SolutionHow many dative bonds are present in BF−4
View Solution

