Suggest reasons why the B–F bond lengths in BF3 (130 pm) and BF–4(143 pm) differ.
Video Solution
Text Solution
The B–F bond length in BF3 is shorter than the B–F bond length in . BF3BF−4 is an electron-deficient species. With a vacant p-orbital on boron, the fluorine and boron atoms undergo pπ–pπ back-bonding to remove this deficiency. This imparts a double- bond character to the B–F bond.
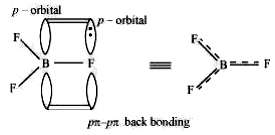
This double-bond character causes the bond length to shorten in BF3 (130 pm). However, when BF3 coordinates with the fluoride ion, a change in hybridisation from sp2
(inBF3) to sp3 (inBF−4) occurs. Boron now forms 4σ bonds and the double-bond character is lost. This accounts for a B–F bond length of 143 pm in BF−4ion.

|
Similar Questions
Knowledge Check
The decreasing order oof bond angles in BF2,NH3,PF3 and I−3 is:
AI−3>NH3>PF3>BF3BBF3>I−3>PF3>NH3CBF3>NH3>PF3>I−3DI−3>BF3>NH3>PF3Back bonding in BF3 does not afect
Aplanarity, lewis acidic strength and bond angleBbond length, hybridisation and bond strengthCbond angle, planarity, geometryDLewis acidity, bond length, bond order (B-F)Statement-1 : The B-F bond length in BF3 is not identical with that in BF−4 because
Statement-2 : Back bonding is involved in BF−4 but not in BF3AStatement -1 is true, Statement-2 is true and statement-2 is correct explanation for statement -1.BStatement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1CStatement-1 is true, statement-2 is falseDStatement-1 is false, statement-2 is true.
B — F bond length in BF3 and [BF4]−are respectively -
View SolutionHow many dative bonds are present in BF−4
View SolutionWhy is BF3 a Lewis acid?
View SolutionCovalent radius of fluorine is 64 pm but the bond length is not equal to 128 pm and that is 143 pm and bond energy is found to be comparable to I2.
View SolutionIn diamond, the C−C bond lengths are ______ pm.
View Solution

