Predict the number of unpaired electrons in square planar [PtCI4]2− ion .
Video Solution
Text Solution
Oxidation state of Pt in [PtCI4]2− hybridisation is required For the avilabitity of one d-orbitals Hence there should be no unpaired electrons [PtCI4]2− ion
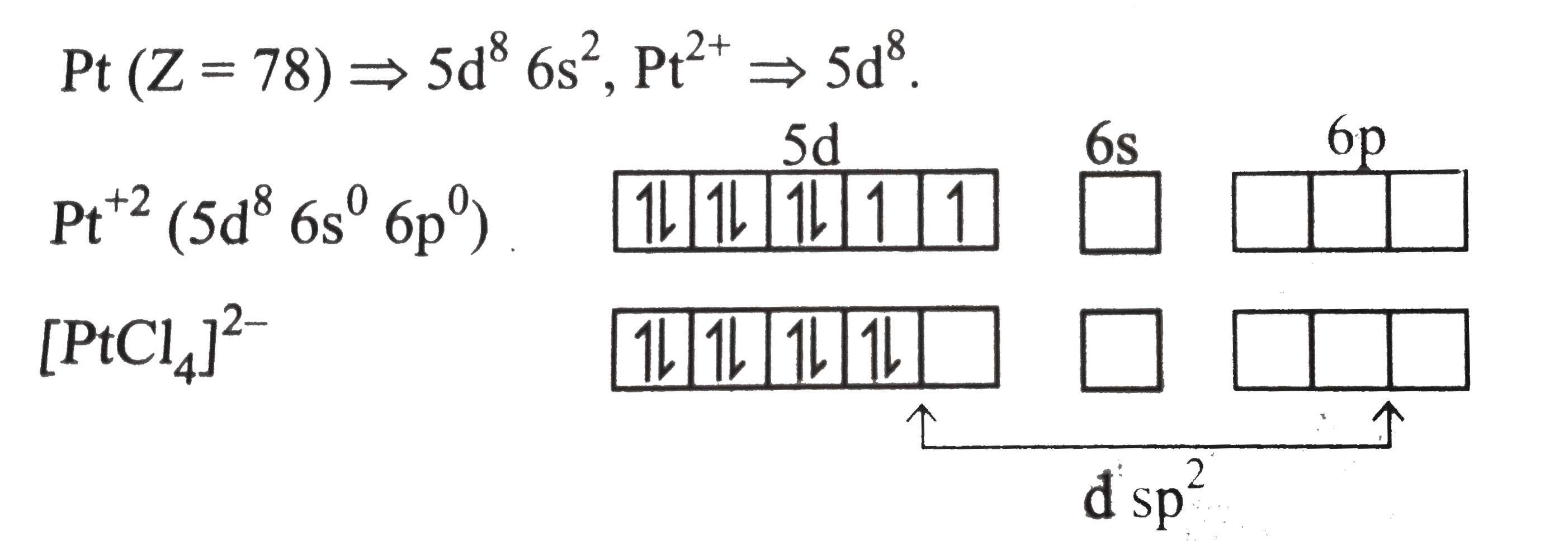
Since CIΘ being weak ligand so pairing of 3d electrons should not occur but for square planar complex (dsp2) hybridisation pairing of electrons in 2 half-filed 5d orbitals occurs .
|
Similar Questions
Knowledge Check
The number of unpaired electrons in the square planar [Pt(CN)4]2− ion is
A2B1C0D3What is (are) number (s) of unpaired electron(s) in the square planar [Pt(CN)4]2− ion ?
AZeroB1C4D6What is (are) number (s) of unpaired electron(s) in the square planar [Pt(CN)4]2− ion ?
A0B1C4D6
Predict the number of unpaired electrons in the square planar [Pt(CN)4]2− ion.
View SolutionPredict the number of unpaired electrons in a square planar d8 ion.
View SolutionPredict the number of unpaired electrons in a square planar d8 ion.
View SolutionThe number of unpaired electrons in ferrous ion is
View SolutionThe number of unpaired electrons in Cr3+ ion is
View Solution
