Predict the number of unpaired electrons in a tetrahedral d6 ion and in a square planar d7 ion .
Video Solution
Text Solution
The correct Answer is:4,1
Electronic distribution in a tetrahedral d6 ion CN=4 Tetrahedral complex
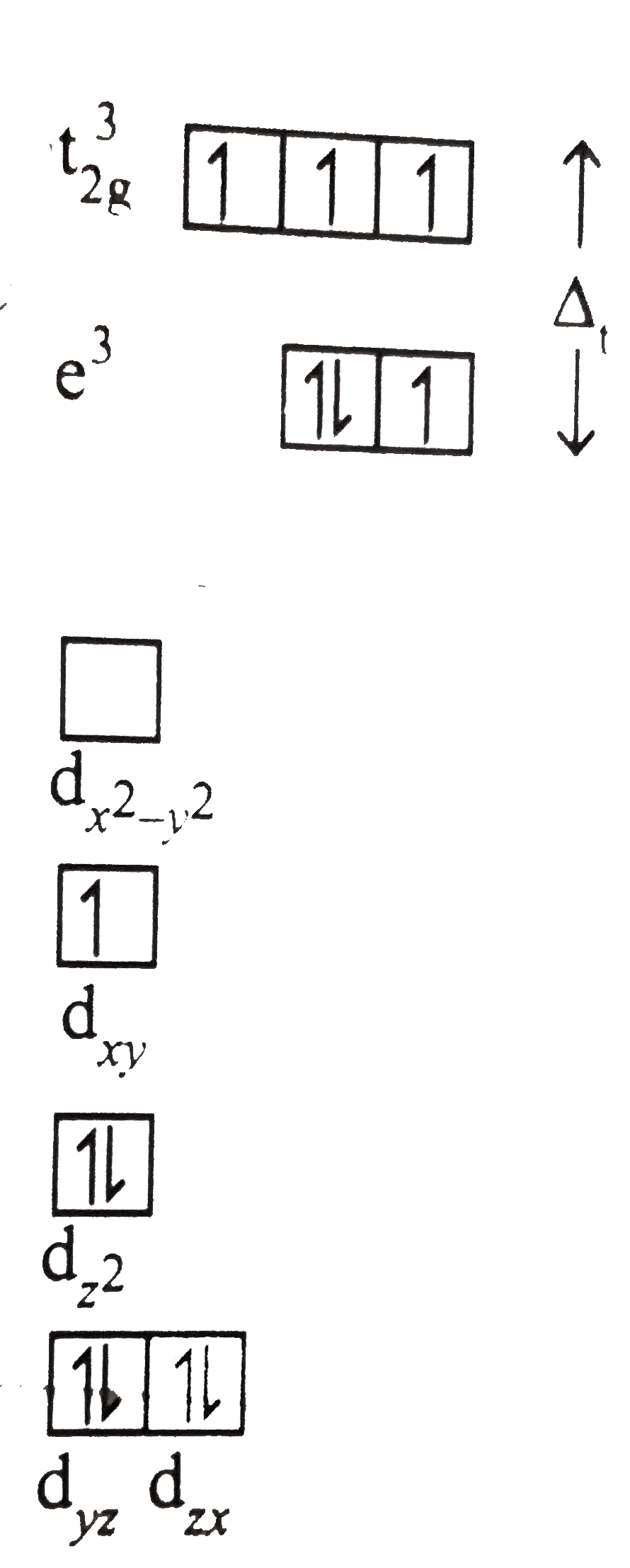
|
Similar Questions
Knowledge Check
The number of unpaired electrons in ferrous ion is
A5B2C4D3The number of unpaired electrons in Cr3+ ion is
A3B5C4D1The number of unpaired electrons in Ce3+ ion is 0
A0B1C2D3
Predict the number of unpaired electrons in square planar [PtCI4]2− ion .
View SolutionNumber of unpaired electrons in Fe2+ ion is
View SolutionThe number of unpaired electron(s) in Mg2+ ion is
View SolutionIon with maximum number of unpaired electrons
View SolutionIon with maximum number of unpaired electrons
View Solution
