Arrange the hydrogen halides in decreasing order of their reducing power.
Video Solution
Text Solution
Reducing power: HI>HBr>HCl>HF
|
Similar Questions
Knowledge Check
Arrange the following hydrogen halides in order of their decreasing reactivity with propene.
AHCl>HBr>HIBHBr>HI>NClCHI>NBr>HClDHCl>HI>HBrArrange the following hydrogen halides in order of their decreasing reactivity with propene.
AHCl gt HBr gt HIBHBr gt HI gt HClCHI gt HBr gt HClDHCl gt HI gt HBrArrange the following in decreasing order of acidity ?
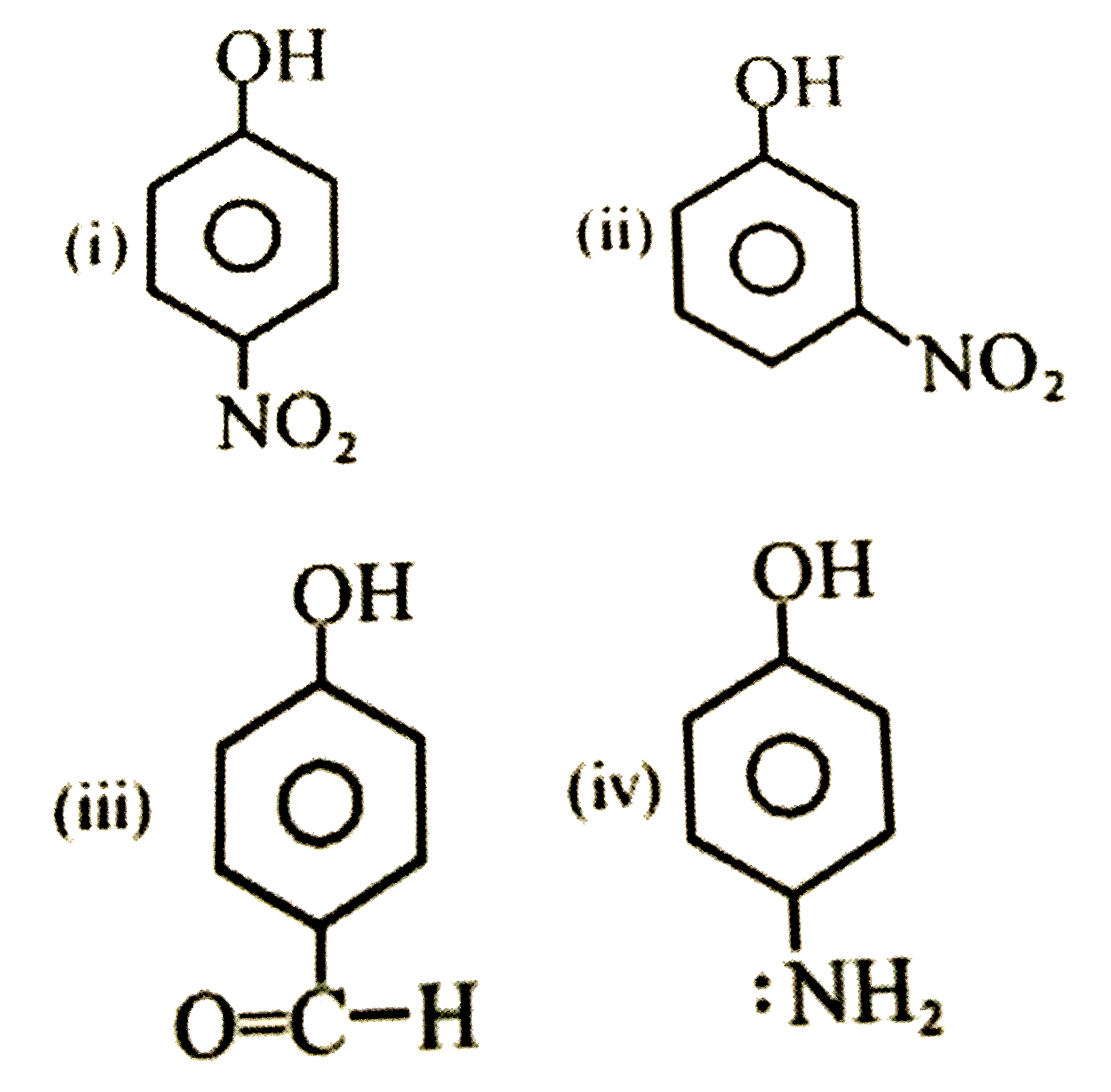 AIV>II>I>IIIBIV>II>III>ICIII>I>II>IVDI>III>II>IV
AIV>II>I>IIIBIV>II>III>ICIII>I>II>IVDI>III>II>IV
Arrange the following in decreasing order of basicity:

View SolutionArrange the following in decreasing order of stability
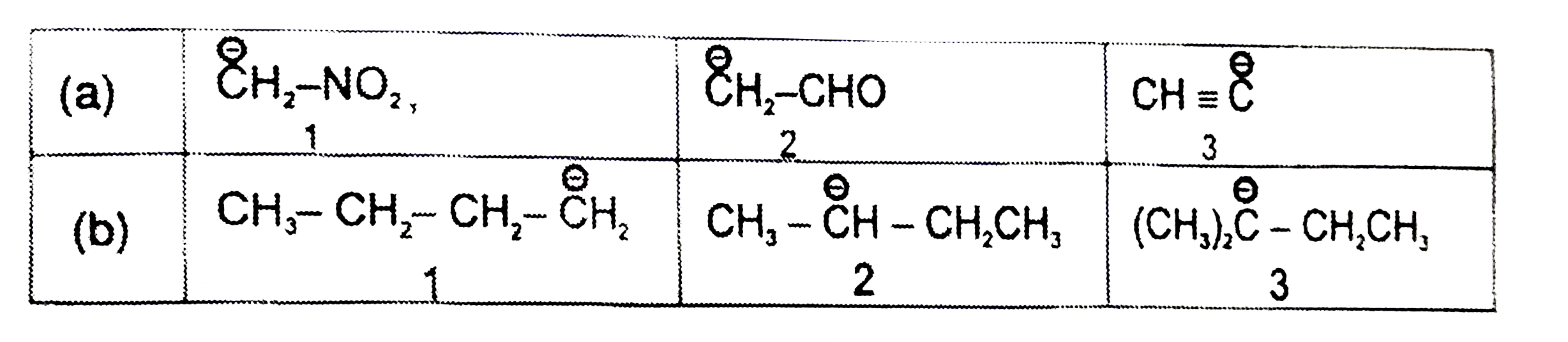
View SolutionArrange the following in decreasing order of stability
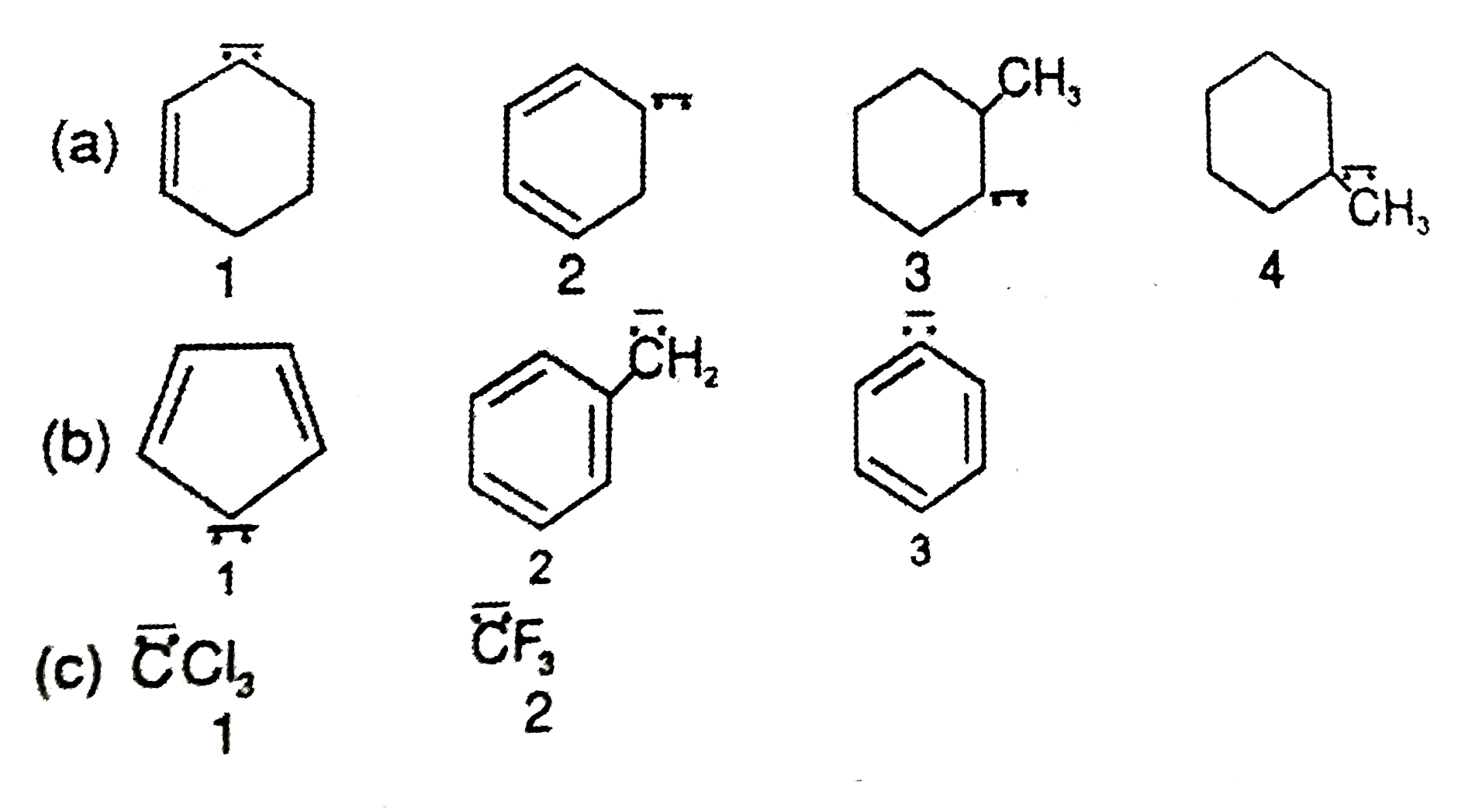
View SolutionArrange the following in decreasing order of stability.
View SolutionArrange the followingg hydrogen halides in order of their decreasin reactivity with propene.
View Solution