Arrange the following hydrogen halides in order of their decreasing reactivity with propene.
A
HCl gt HBr gt HI
B
HBr gt HI gt HCl
C
HI gt HBr gt HCl
D
HCl gt HI gt HBr
Video Solution
Text Solution
The correct Answer is:C
Reactivity increases as the strength of H-X bond decreases, i.e., HI gt HBr gt HCl
|
Topper's Solved these Questions
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (MULTIPLE CHOICE -II)
Explore 9 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (SHORT ANSWER QUESTIONS)
Explore 20 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:NCERT QUESTION AND EXERCISES WITH ANSWERS (NCERT EXCERCISES)
Explore 24 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBook:PRADEEPChapter:EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESExercise:Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
Explore 10 Videos
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
Explore 15 Videos
Similar Questions
Knowledge Check
Arrange the followingg hydrogen halides in order of their decreasin reactivity with propene.
AHClgtHBrgtHIBHBrgtHIgtHClCHIgtHBrgtHClDHClgtHIgtHBrArrange the following compounds in order of their decreasing reactivity with an electrophile,E(+)
i) Chlorobenzene
ii) 2,4- dinitrochlorobenzene
iii)p- nitrochlorobenzeneAC gt B gt ABB gt C gt ACA gt C gt BDA gt B gt CArrange these in correct order of decreasing reactivity
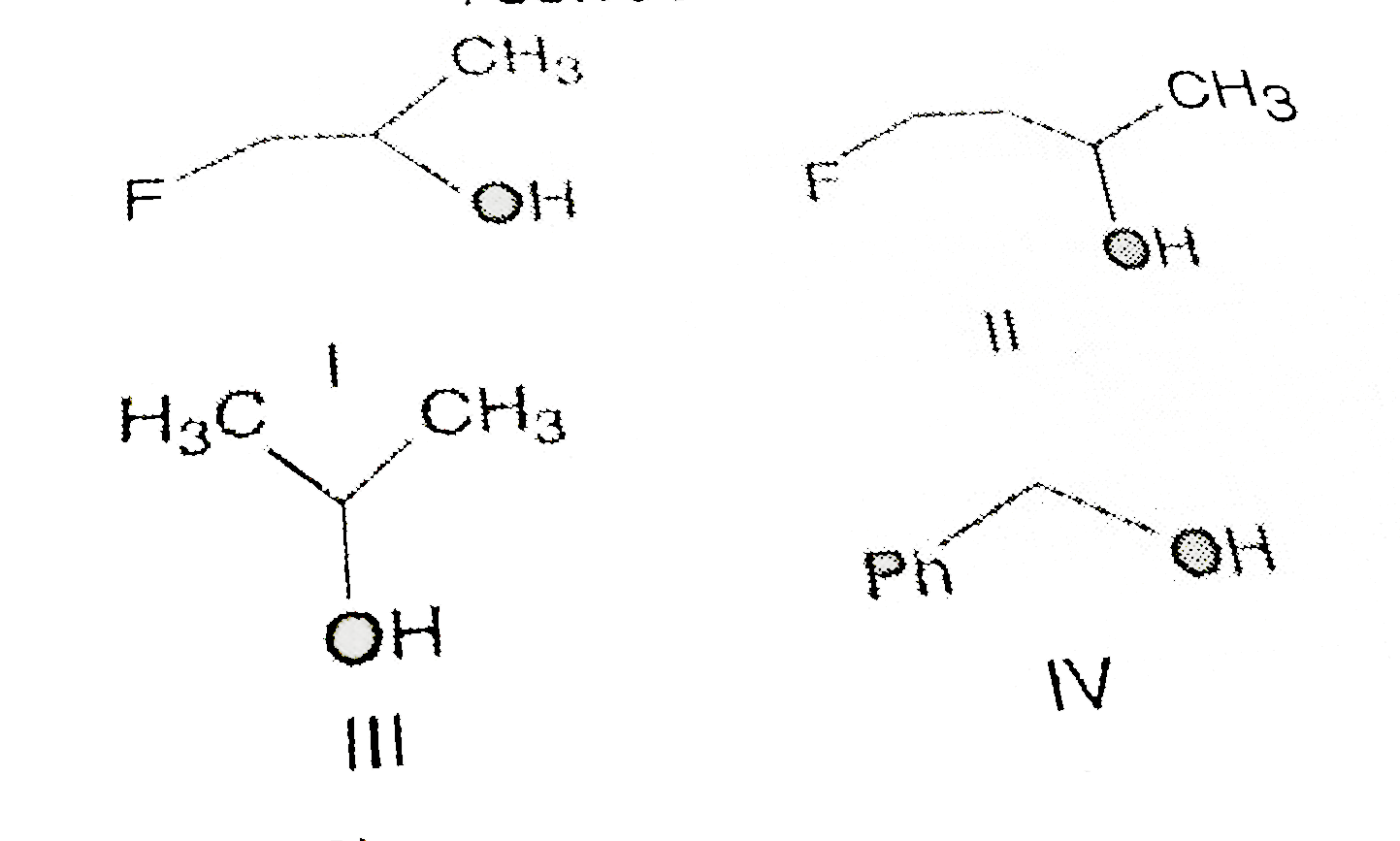 AI>II>III>IVBI>III>II>IVCIV>III>II>IDIV>III>I>II
AI>II>III>IVBI>III>II>IVCIV>III>II>IDIV>III>I>II
Arrange the following in decreasing order of stability.
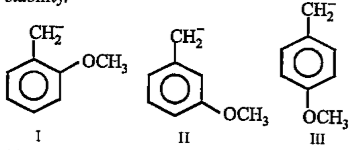
View SolutionArrange the hydrogen halides in decreasing order of their reducing power.
View SolutionArrange the following in decreasing order of basicity:

View SolutionArrange the following compounds in order of decreasing reactivity in the elimination (bimolecular ) reaction with C2H5ONa
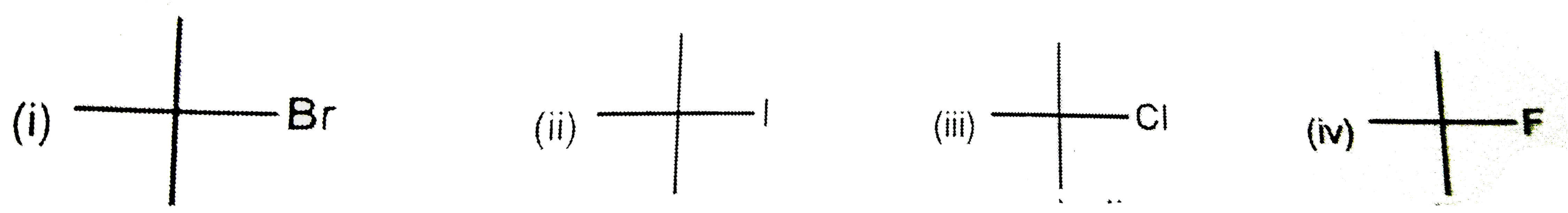
View SolutionArrange these in correct order of decreasing reactivity.
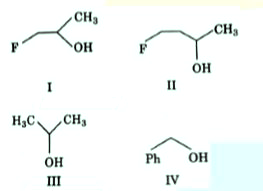
View Solution
