Why pyridine is a much weaker base than aliphatic amines ?
Pyridine 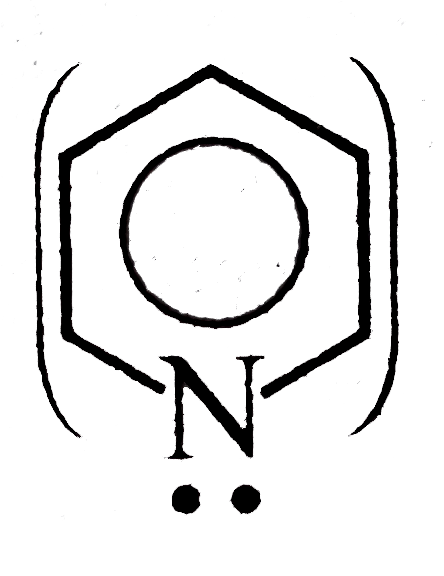 is a weaker base than R(##KSVORGP1C04E01007_S02##)NH2 due to +I effect in aliphatic amine (aliphatic amine gt aromatic amine).
is a weaker base than R(##KSVORGP1C04E01007_S02##)NH2 due to +I effect in aliphatic amine (aliphatic amine gt aromatic amine).
Similar Questions
(i) What is Gabriel phthalimide synthesis? Why aromatic primary amines cannot be prepared by this method?
(ii) Why are aromatic amines weaker bases than aliphatic amines?
View SolutionAccount for any two of the following :
(a) Amines are basic substances while amides are neutral.
(b) Aromatic amines are weaker bases than aliphatic amines.
View Solution
Knowledge Check
Why o-derivative is weaker base than aniline?
AagtbgtcgtdBdgtcgtbgtaCcgtbgtagtdDagtcgtbgtd
Why are alcohols weaker acids than water?
View SolutionAniline is a weaker base than cyclohexylamine. Explain.
View SolutionWhy is carbon nitrogen bond length in aromatic amines shorter than in aliphatic amines?
View SolutionWhy is carbon-nitrogen bond length in aromatic amines shorter than in aliphatic amines?
View SolutionGive reason: Aniline is a weaker base than cyclohexylamine.
View Solutionp-methoxyaniline is a stronger base than aniline but p-nitroaniline is a weaker base than aniline. Explain.
View SolutionAssertion : p-nitroaniline is a weaker base than p-toluidine.
Reason: The electron donating −NO2 group in p-nitroaniline makes it a weaker base.
View Solution
