The decreasing order of acidic character of the following is :
(I) CH3SH
(II) CH3OH
(III) H2O
(IV) EtOH.
I>II>III>IV
IV>III>II>I
I>III>II>IV
III>I>II>IV
The correct Answer is:A
(a) Thiols (RSH) are stronger acid than alcohols Methanol (pKa=15.5) is a stronger acid than H2O(pKa=15.7), but other alcohols are weaker than H2O. So (a) has acidic character.
Similar Questions
The decreasing order of acidic character of the following is :
(I) p-Nitrophenol
(II) o-Nitrophenol
(III) m-Nitrophenol
(IV) Phenol.
View SolutionExplain the following :
(a) In gaseous phase, the acidic characters of the following are :
(i) CH3CH2COOH(I)>CH3COOH(II)
(ii) ClCH2COOH(III)>FCH2COOH(IV)
(iii) Me3C−OH(V)>MeCH2CH2CH2OH(VI).
(b) Account for the following differences in the acidic characters of the following :
(i) (I)Me3C−CH2−COOH>(II)Me3SICH2COOH
(ii) (III)H2C=CHCH2COOH>(IV)CH3CH2COOH
(iii) (V)HC≡C−CH2COOH(pKa3.22)>(VI)H2C=CHCH2COOH(pKa=4.35)
(iv) CH3−C∣∣O−CH2−COOH>
(v) (IX)N≡C−CH2COOH>(X)CH3COCH2−COOH
(vi) (XI)PhCh2COOH>(XII)CH2=CHCH2COOH.
View Solution
Knowledge Check
The decreasing order of acidic character of the following is :
(I) CH=CH
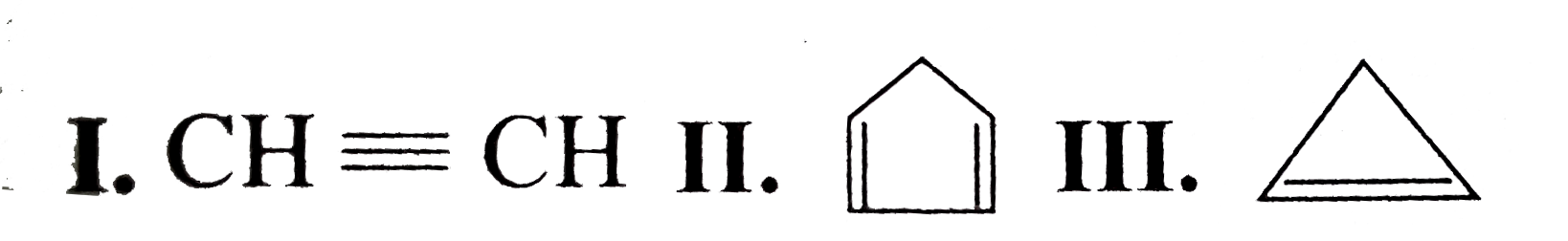 .AI>II>IIIBII>I>IIICIII>II>IDI>III>II
.AI>II>IIIBII>I>IIICIII>II>IDI>III>IIThe decreasing order of acidic character of the following is:
(I)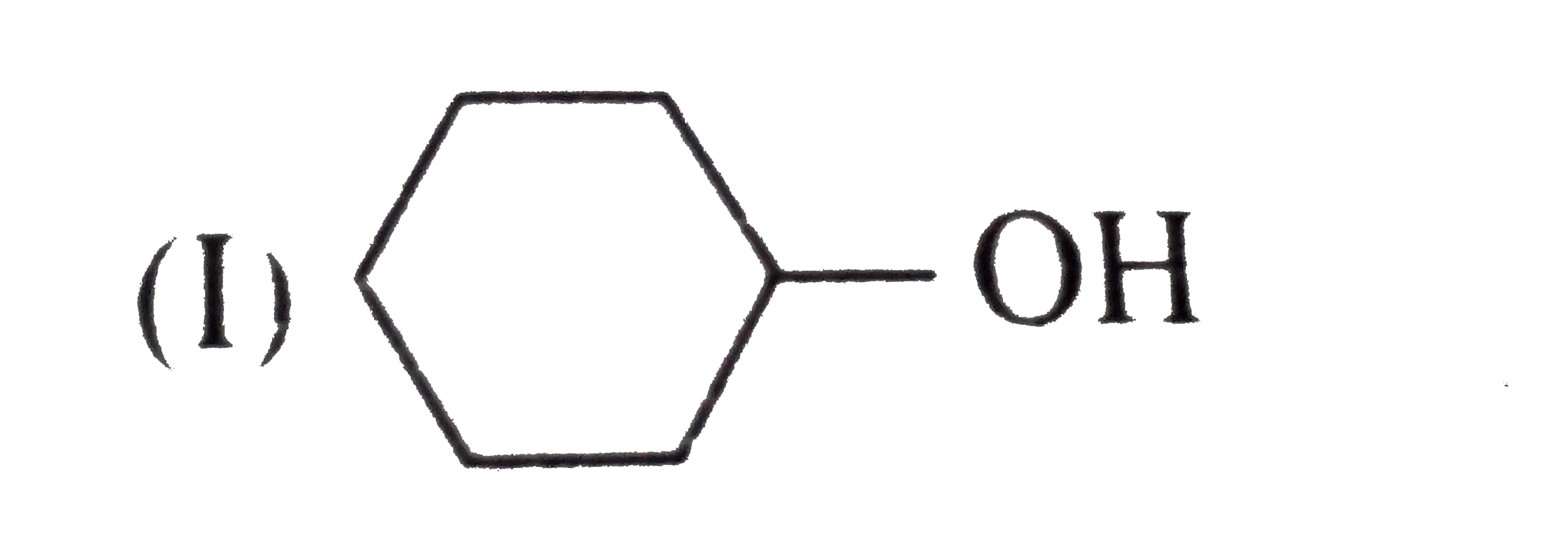 (II)
(II) 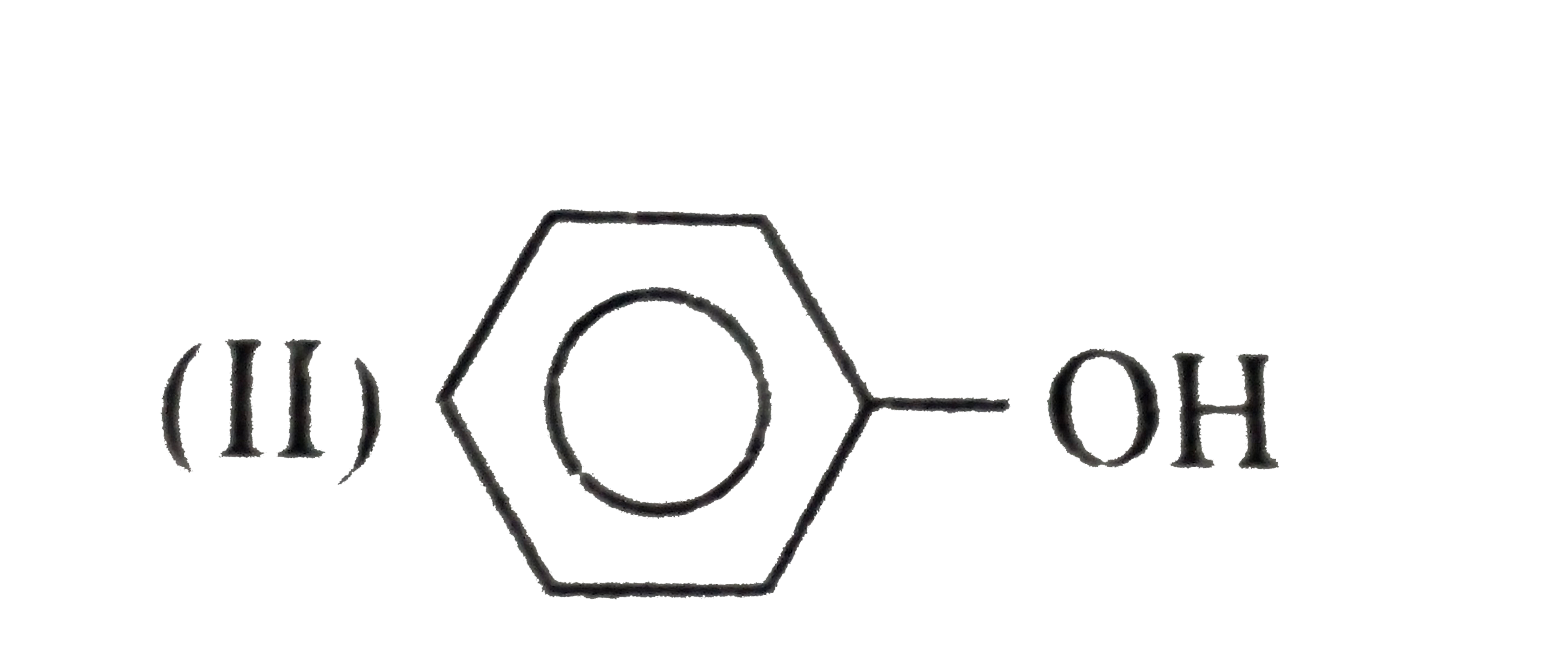
(III)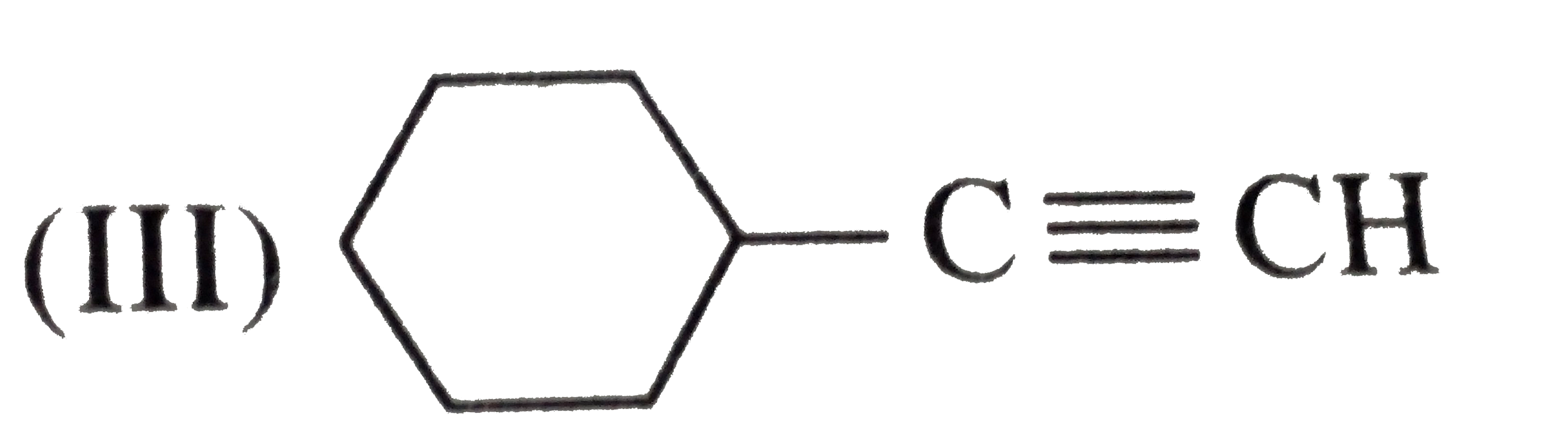 (IV)
(IV) 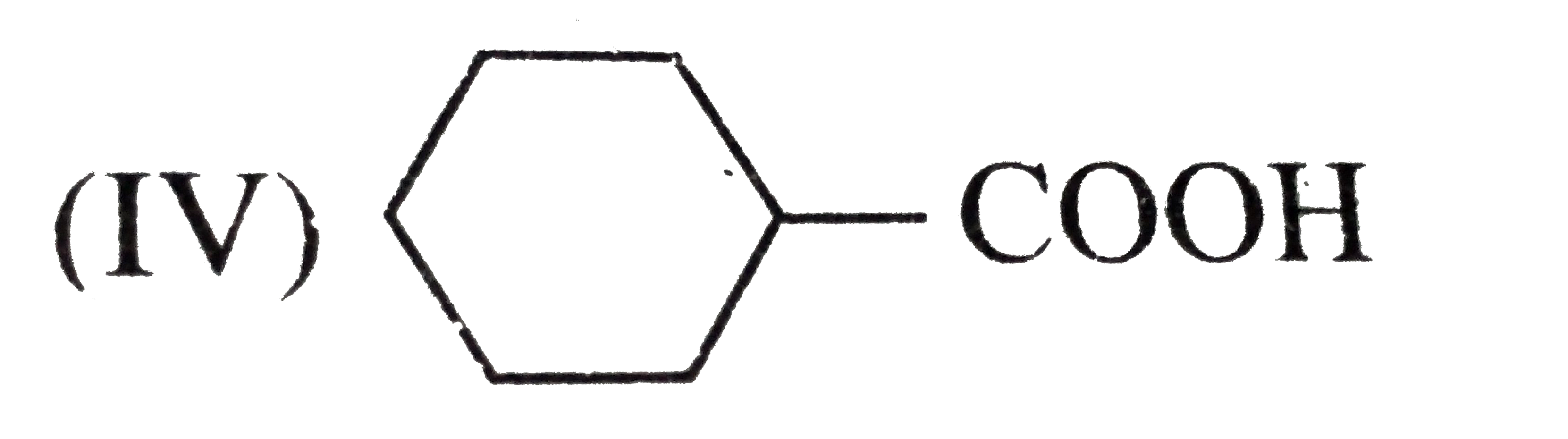 AII>IV>III>IBI>III>IV>IICIV>II>I>IIIDIII>I>II>IV
AII>IV>III>IBI>III>IV>IICIV>II>I>IIIDIII>I>II>IVThe decreasing order of the basic character of the following is :
(I) F^(Ө)
(II) NOӨ3
(III) H2O
(IV) CH3OH.A(II)>(I)>(IV)>(III)B(III)>(IV)>(I)>(II)C(I)>(II)>(III)>(IV)D(IV)>(III)>(II)>(I)
The decreasing order of +I effect of the following is :
(I) −OӨ
(II) Me−
(III) Et−
(IV) Me2CH−
(V) Me3C−.
View SolutionThe decreasing order of acidic character of the following is:
(I) EtOH
(II) Me−COOH
MeOOC−H2−COOMe
(IV)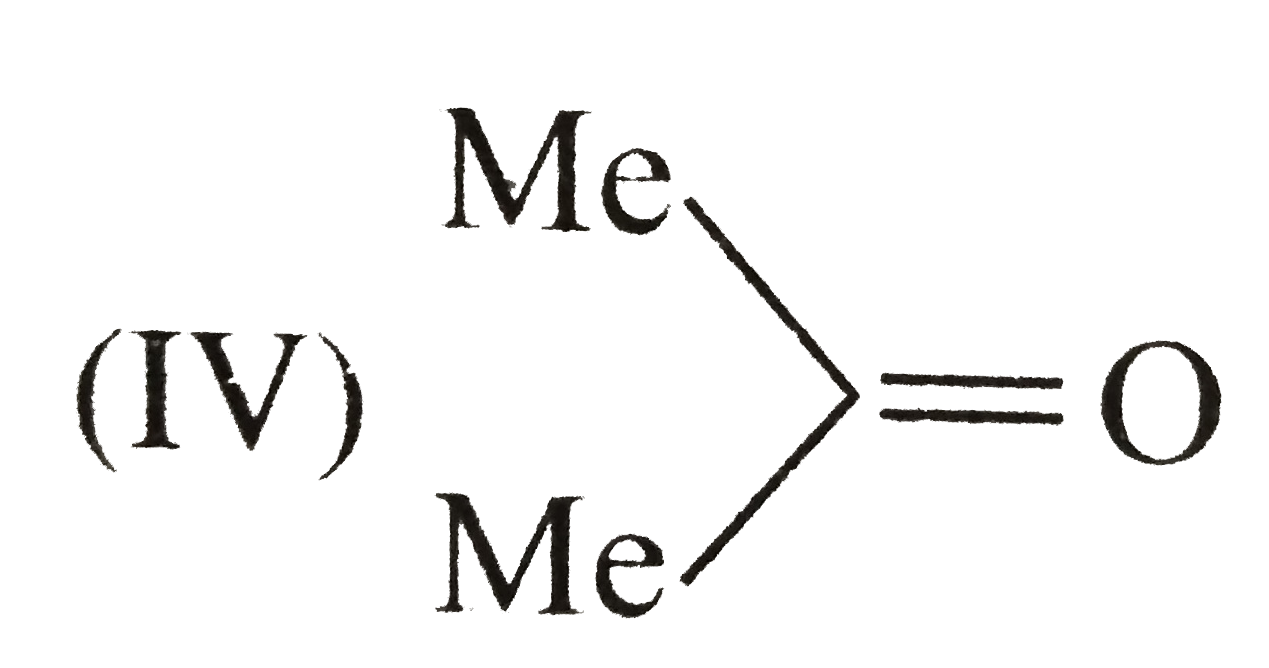
View SolutionThe decreasing nucleophilicity of the following is :
(I) CH3SӨ
(II) CH3OӨ
(III) ӨOH
(IV) EtOӨ.
View SolutionThe decreasing basic character of the following is :
(I) PhOӨ
(II) CH3SӨ
(III) ӨOH
(IV) MeOӨ.
View SolutionIsoelectronic structures among the following structures are :
(I)CH+3,(II)H3O+(III)NH3,(IV)CH−3.
View Solution
