i. 27.8gm mixture of alkyne and alkane (both containing same number of carbon atoms) is dissolved in 1000gm of benzene. The solution freezes at 2.45∘C (lower than that of benzene). Another 27.8gm mixture requires 0.6 mol of H2 for complete hydrogenation. Calculate the chemical formula of alkyne and alkane (Kf for C6H6=4.9).
ii. Alkyne on hydrogenation with H2+Pt gives the same alkane.
Alkyne does not react with ammoniacal AgNO3 solution. Give the structures of both alkyne and alkane.
Video Solution
Text Solution
i. Formula of alkyne =CnH2n−2, Alkane =CnH2n+2
(Same number of C atoms).
Let weight of alkyne = xgm and weight of alkane
=27.8−xgm
Molecular weight (MW) of alkyne
=(12n+2n−2)=(14n−2)
Molecular weight (MW) of alkane
=(12n+2n+2)=(14n+2)
R−C≡C−R2H2/Ni−−−−→R−CH2−CH2−R
2 mol of H2=1 mol of alkyne
0.6 mol of H2=0.3 mol of alkyne
∴0.6 mol of H2⇒xgm of alkyne
2 mol of H2⇒x0.6×2=x0.3gm of alkyne
=MW of alkyne
∴x0.3=(14n−2) ∴ξmplies0.3(14n−2) ....(i)
ΔTf=Kf(m1+m2) (Let m1⇒ molality of alkyne, m2⇒ molality of alkane)
2.45=4.9(m1+m2)
∴m1+m2=2.454.9=0.5
∴m1+m2=0.5
m1=Moles of alkyne×1000W1(wt.of solvent)=0.3×10001000
m1=0.3,m1+m2=0.5 ∴m2⇒0.2
m2=Wt.of alkane×1000Mof alkane×1000=0.2=27.8−8(14n+2)
Substitute the value of x from (i).
0.2=27.8−0.3(14n−2)(14n+2), solving we get n=4
Formula of alkyne =C4H6
Formula of alkane =C4H10
ii. Alkyne is internal as it does not react with ammoniacal AgNO3 solution [Ag(NH3)2]⊕ӨOH.
So the structures of alkyne and alkanes are:
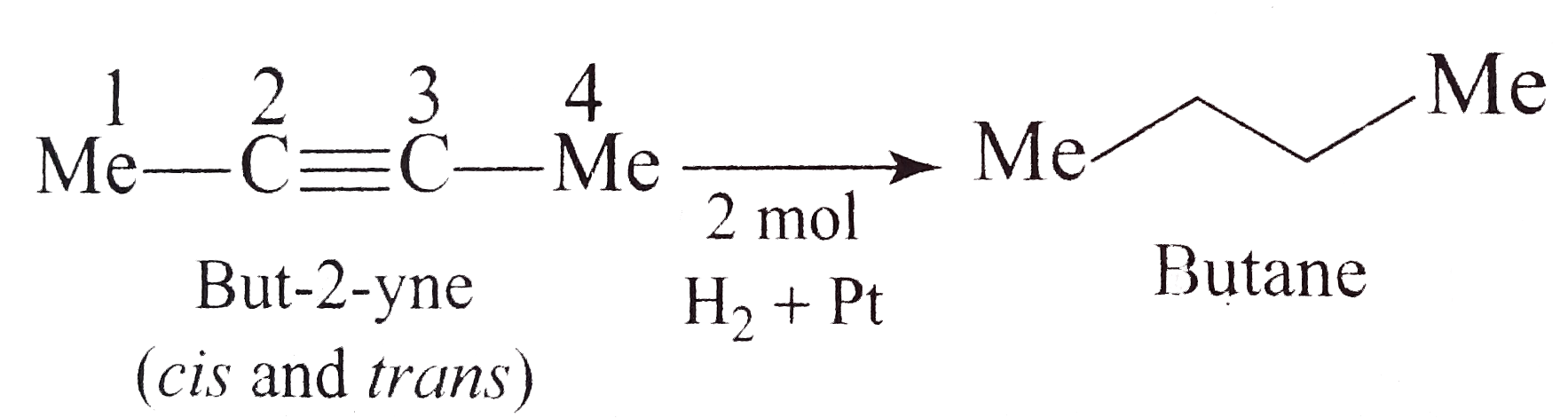
|
Topper's Solved these Questions
- ALKYNESBook:CENGAGE CHEMISTRYChapter:ALKYNESExercise:Exercises (Subjective Type)
Explore 31 Videos
- ALKYNESBook:CENGAGE CHEMISTRYChapter:ALKYNESExercise:Exercises (Linked Comprehension Type)
Explore 51 Videos
- ALKYNESBook:CENGAGE CHEMISTRYChapter:ALKYNESExercise:Exercises (Archives - Analytical and Desriptive Type)
Explore 4 Videos
- ALKENES AND ALKADIENESBook:CENGAGE CHEMISTRYChapter:ALKENES AND ALKADIENESExercise:Single correct Answer
Explore 14 Videos
- APPENDIX - INORGANIC VOLUME 1Book:CENGAGE CHEMISTRYChapter:APPENDIX - INORGANIC VOLUME 1Exercise:chapter-7 Single correct answer
Explore 1 Video
Similar Questions
Knowledge Check
Hydrogenation of alkyne is
AAddition reactionBElimination reactionCRearrangement reactionDSubstitution reactionWhich of the following alkynes will not reacts with ammoniacal CuCl?
Abut-1-yneBbut-2-yneCpropyneDethyneAn alkyne has 8 carbon atoms. The number of hydrogen atoms in it are
A8B16C18D14
An alkane C6H14 gives onlt two monochloroalkanes on chlorination. What is the structure of the original alkane ?
View SolutionThe general formula of alkyne is CnH2n. True/false
View SolutionThe catalyst used to convert alkanes containing 6 to 10 carbon atoms into benzene and its homologous at nearly 600°C are
View Solution20 ml of a gaseous alkyne exactly requires 80 ml O2 for complete combustion . The alkyne is :
View SolutionCompound (X) on complete catalytic hydrogenation with H2/Pt gives an alkane. The number of moles of H2 required per mole of compound (X) is:
View Solution
