Reactivity of MeMgBr with the following in the decreasing order is :
(i) 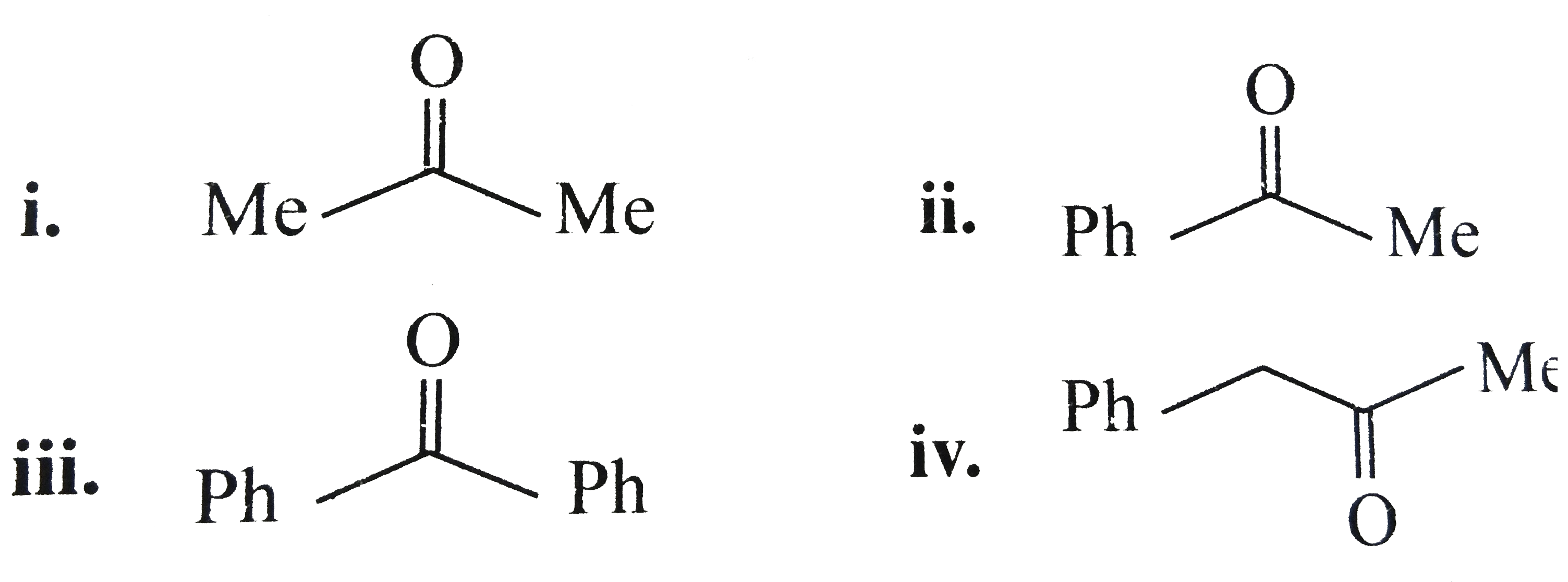
A
(i)>(ii)>(iii)>(iv)
B
(iv)>(i)>(ii)>(iii)
C
(i)>(iii)>(ii)>(iv)
D
(iv)>(ii)>(iii)>(i)
Video Solution
Text Solution
The correct Answer is:B
(b) The order of reactivity of G.R with different functional groups is aliphatic aldehyde gt aromatic aldehyde gt aliphatic ketone gt aromatic ketone. Also, EWG favours NA reaction.
(iv) is aliphatic ketone with −I effect of Ph group .
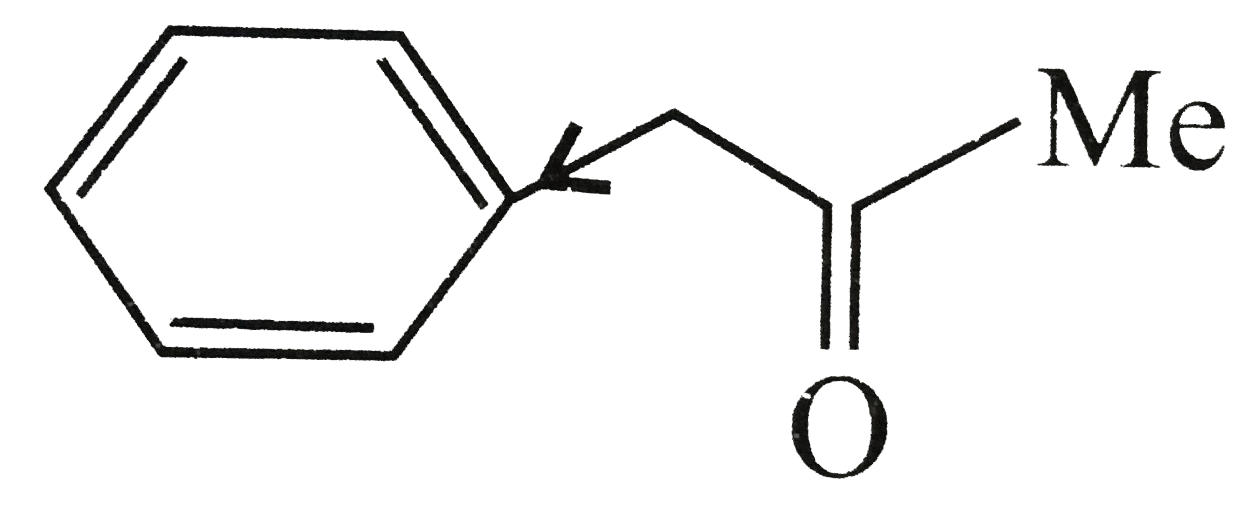 (+R effect is not possible since the double bond of benzene ring is not in conjugation with (C=O) group. So (iv) is the most reactive ketone.
(+R effect is not possible since the double bond of benzene ring is not in conjugation with (C=O) group. So (iv) is the most reactive ketone.
Hence, the order is iv>i>ii>iii.
|
Similar Questions
Knowledge Check
Reactivity of PhMgBr with the following in the decreasing order is :
(i) PhCHO
(ii)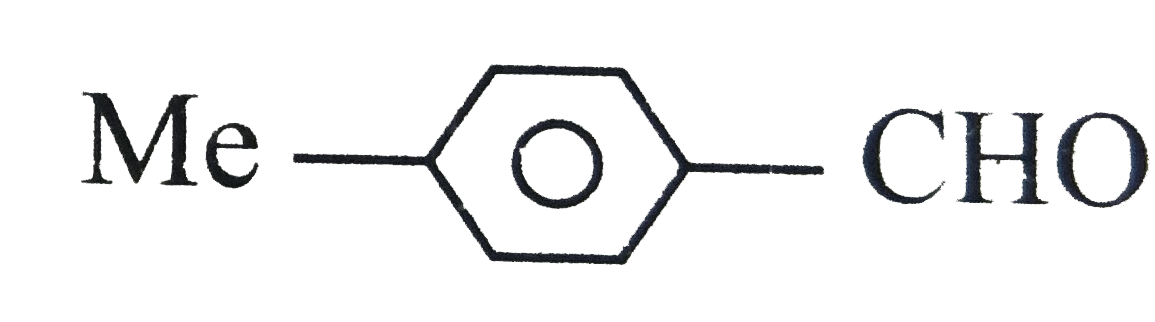
(iii)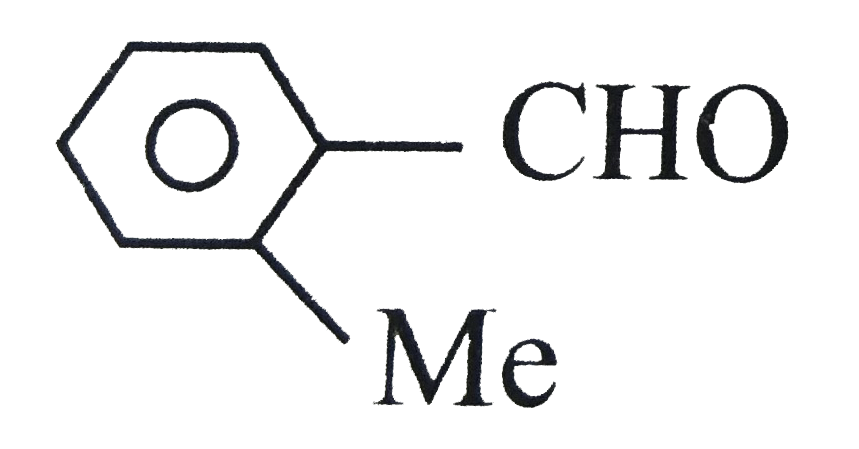
(iv)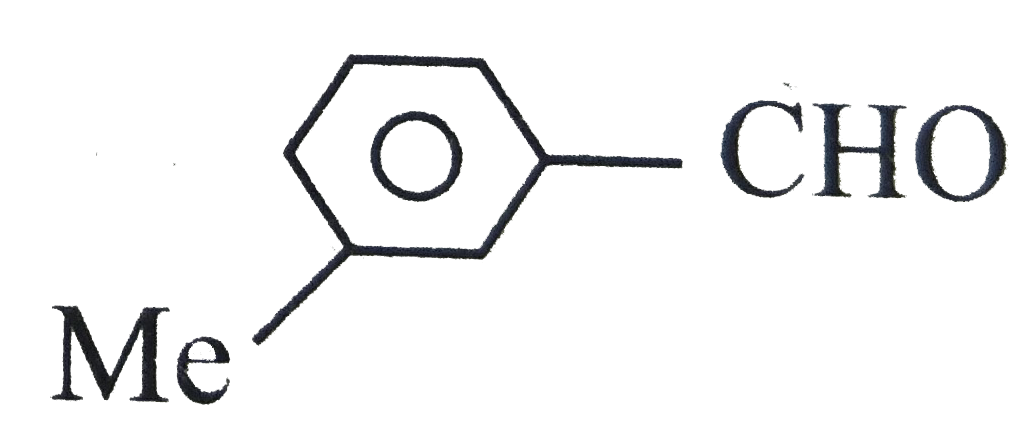 .A(i)>(iv)>(ii)>(iii)B(iii)>(ii)>(iv)>(i)C(i)>(iii)>(ii)>(iv)D(i)>(ii)>(iii)>(iv)
.A(i)>(iv)>(ii)>(iii)B(iii)>(ii)>(iv)>(i)C(i)>(iii)>(ii)>(iv)D(i)>(ii)>(iii)>(iv)Reactivity of PrMgBr with the following in the decreasing order is :
(i) Alcohol
(ii) Aldehyde
(iii) Ketone
(iv) Ester.A(iv)>(iii)>(ii)>(i)B(i)>(ii)>(iii)>(iv)C(ii)>(iii)>(i)>(iv)D(iv)>(i)>(iii)>(ii)Reactivity of EtMgBr with the following in the decreasing order is :
(i) HCHO
(ii) MeCHO
(iii) MeCOMe
(iv) Cl3C−CHO.A(i)>(ii)>(iii)>(iv)B(iv)>(iii)>(ii)>(i)C(iv)>(i)>(ii)>(iii)D(iii)>(ii)(i)>(iv)
Arrange the following in the decreasing order of their boiling points:
I.
View SolutionArrange the following metals in the decreasing order of reactivity:
View SolutionArrange the following metals in the decreasing order of reactivity:
View SolutionIn Wurtz reaction of alkyl halides, the reactivity of alkyl halides follows the decreasing order,
View SolutionThe order of reactivity of phenyl magnesium bromide with the following compounds is :
(I)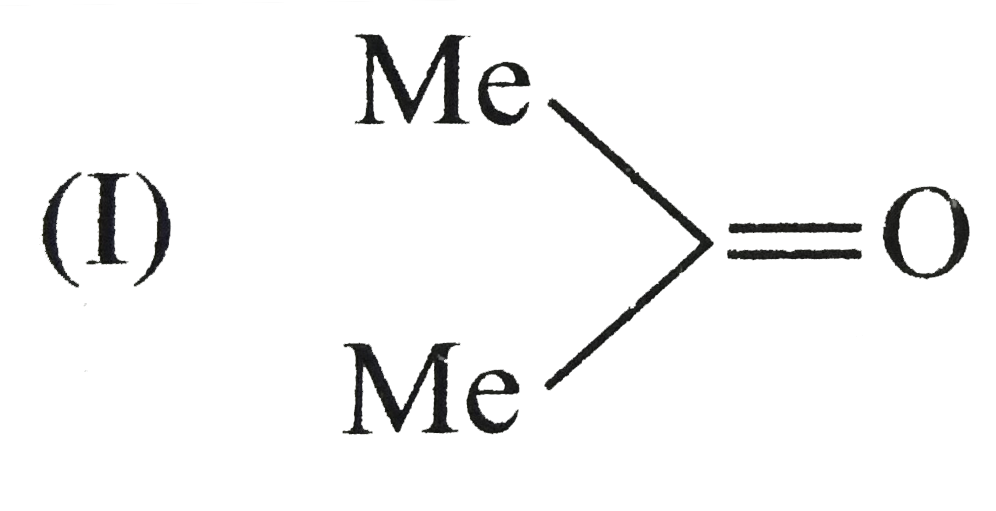
(II)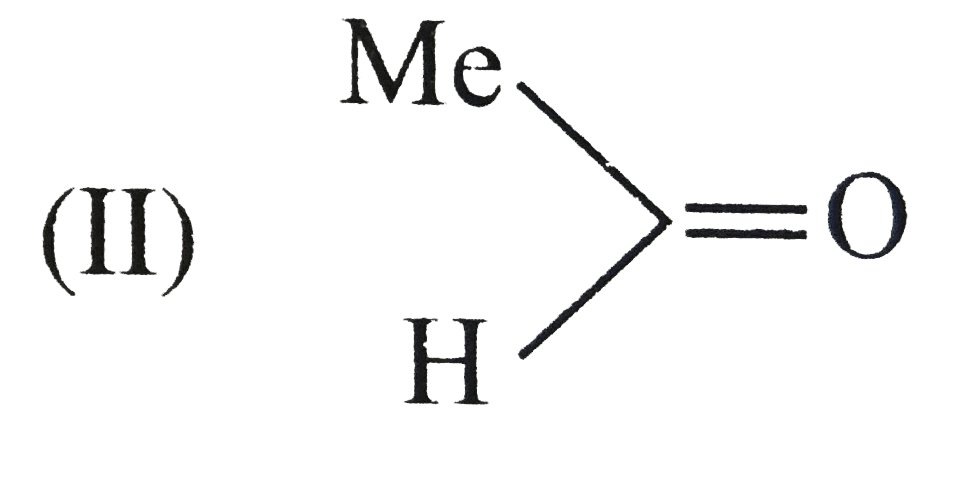
(III)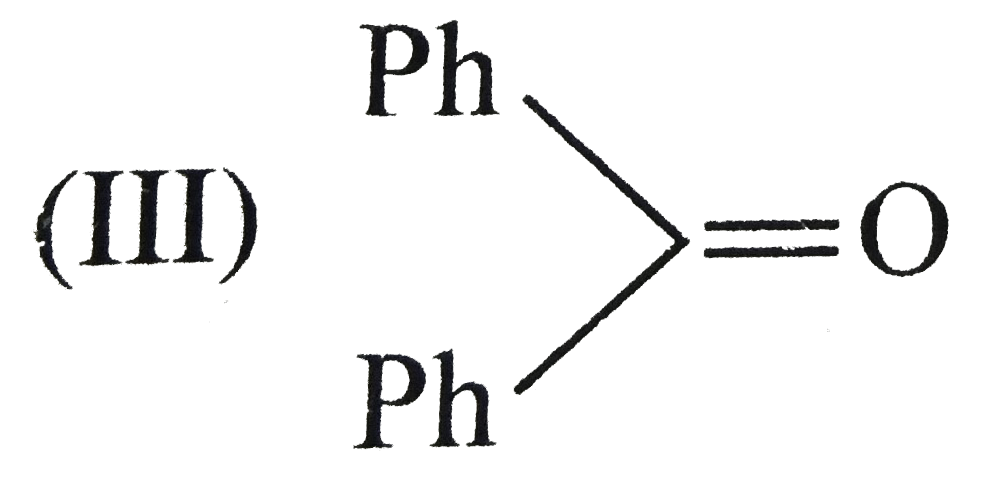 .
.
View Solution


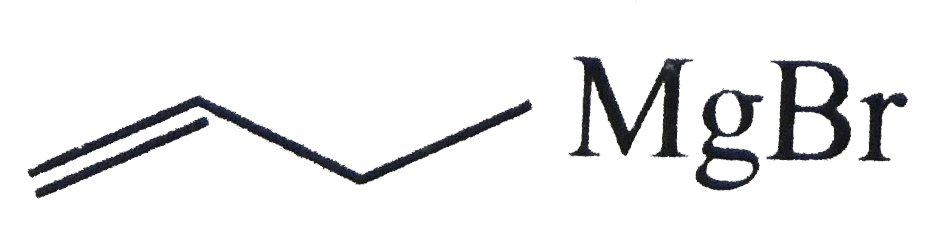
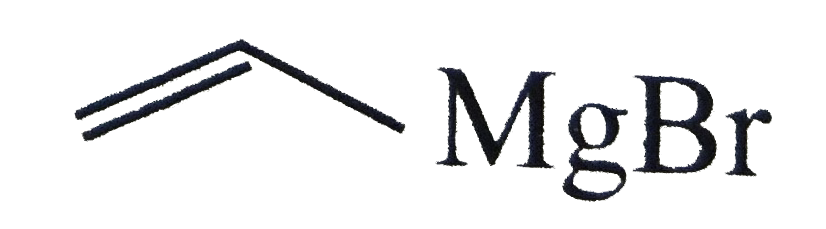 .
.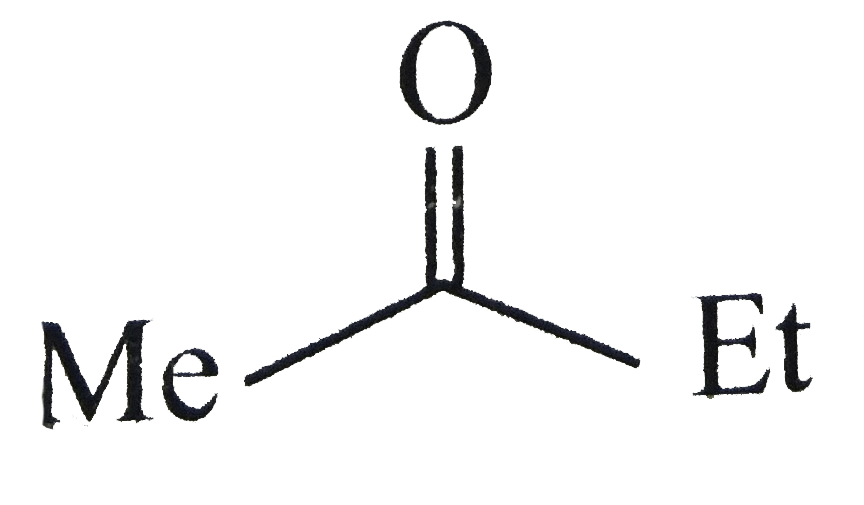 with the following
with the following