Reactivity of PhMgBr with the following in the decreasing order is :
(i) PhCHO
(ii) 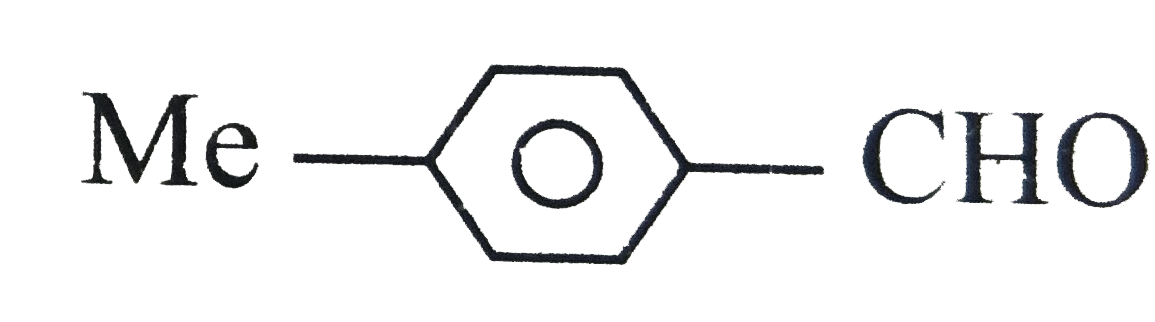
(iii) 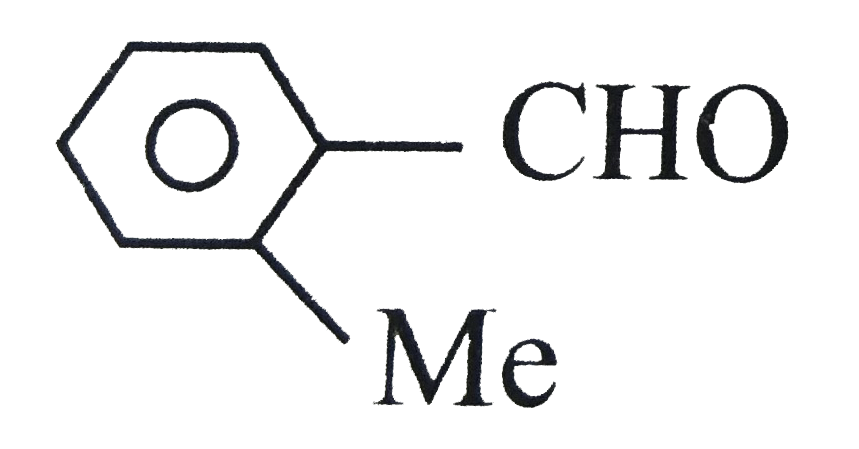
(iv) 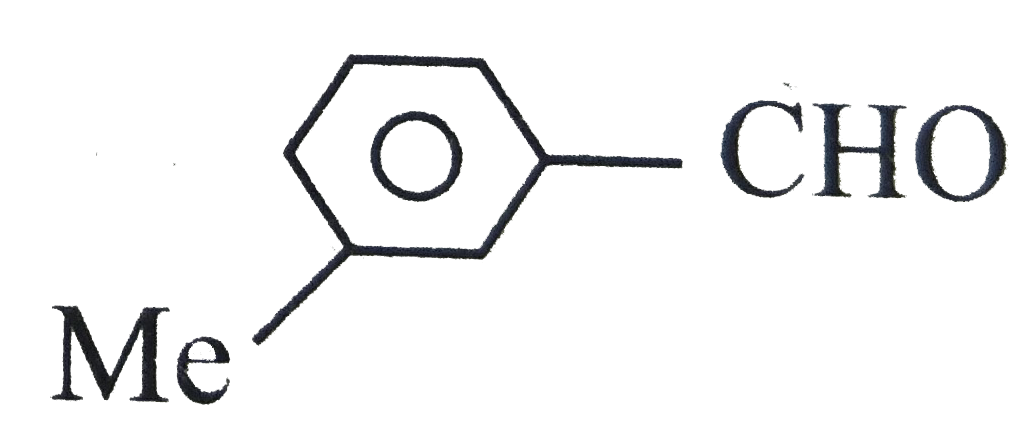 .
.
A
(i)>(iv)>(ii)>(iii)
B
(iii)>(ii)>(iv)>(i)
C
(i)>(iii)>(ii)>(iv)
D
(i)>(ii)>(iii)>(iv)
Video Solution
Text Solution
The correct Answer is:A
(a) EDG (Me group) at o−,m−, and p−positions : At orthoposition, ED power of (Me−) group is more than p and m (due to +I effect and hyperconjugation effect). ED power of (Me−) group at p-position is slightly less than the Me group at ortho-position (due to less +I effect, but equal hyperconjugative effect).
So the order of ED power of (Me−) group is o>p>m−NA is favoured by less-substituted EDG or more-substituted EWG. So, the order of reactivity of PhMgBr with aromatic aldehyde is
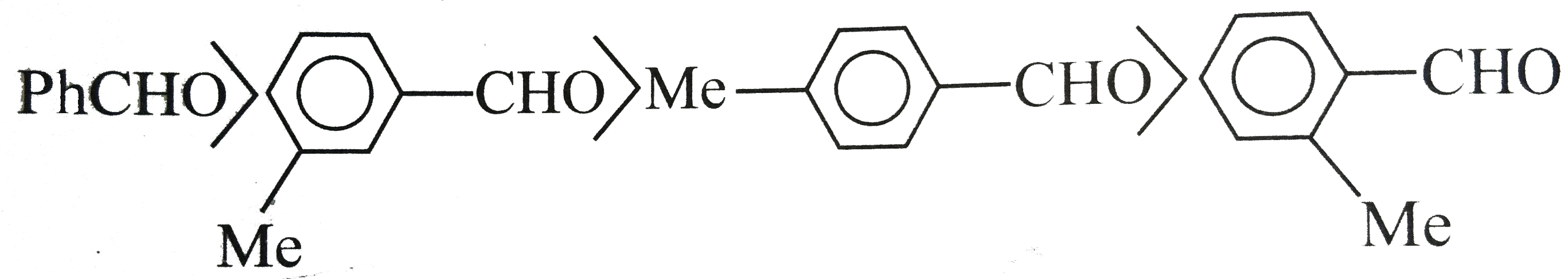
Or (i>iv>ii>iii).
|
Similar Questions
Knowledge Check
Reactivity of MeMgBr with the following in the decreasing order is :
(i)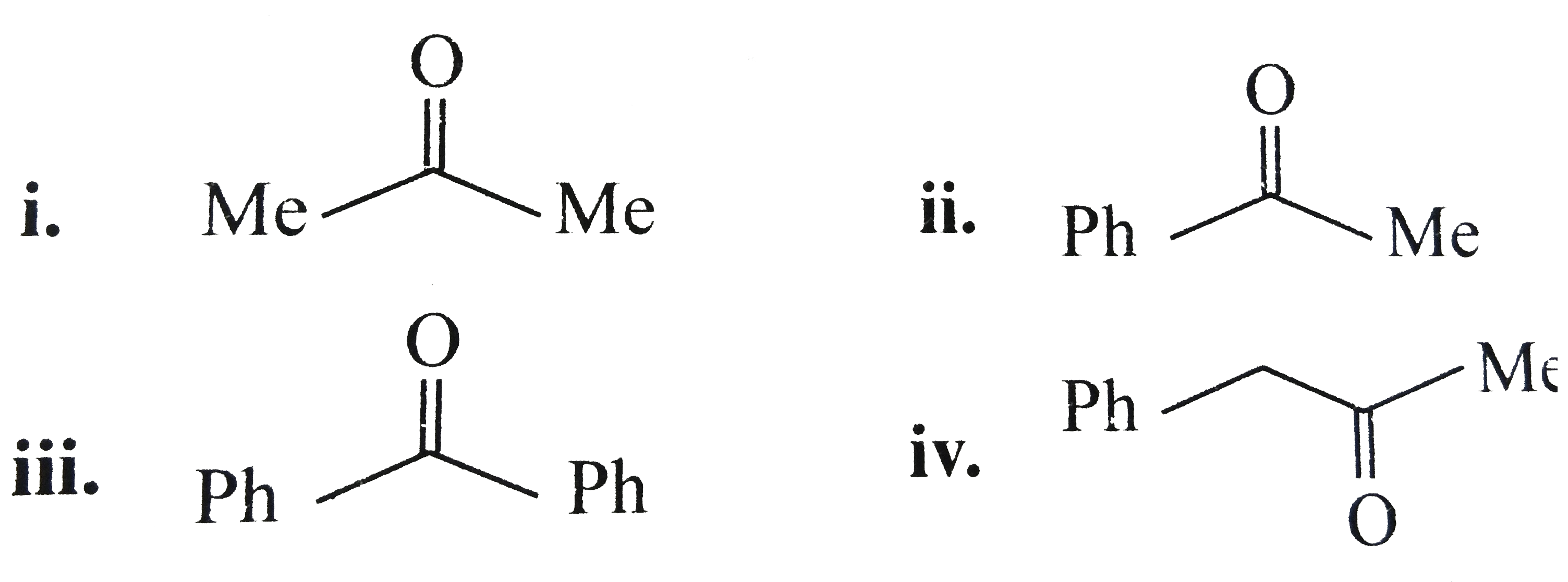 A(i)>(ii)>(iii)>(iv)B(iv)>(i)>(ii)>(iii)C(i)>(iii)>(ii)>(iv)D(iv)>(ii)>(iii)>(i)
A(i)>(ii)>(iii)>(iv)B(iv)>(i)>(ii)>(iii)C(i)>(iii)>(ii)>(iv)D(iv)>(ii)>(iii)>(i)Reactivity of EtMgBr with the following in the decreasing order is :
(i) HCHO
(ii) MeCHO
(iii) MeCOMe
(iv) Cl3C−CHO.A(i)>(ii)>(iii)>(iv)B(iv)>(iii)>(ii)>(i)C(iv)>(i)>(ii)>(iii)D(iii)>(ii)(i)>(iv)Reactivity of PrMgBr with the following in the decreasing order is :
(i) Alcohol
(ii) Aldehyde
(iii) Ketone
(iv) Ester.A(iv)>(iii)>(ii)>(i)B(i)>(ii)>(iii)>(iv)C(ii)>(iii)>(i)>(iv)D(iv)>(i)>(iii)>(ii)
The reactivity of PhMgBr with the following compounds is :

View SolutionReactivity of HCHO with the following Grignard reagent in the decreasing order is
(1) PhMgBr
(2) PhCH2MgBr
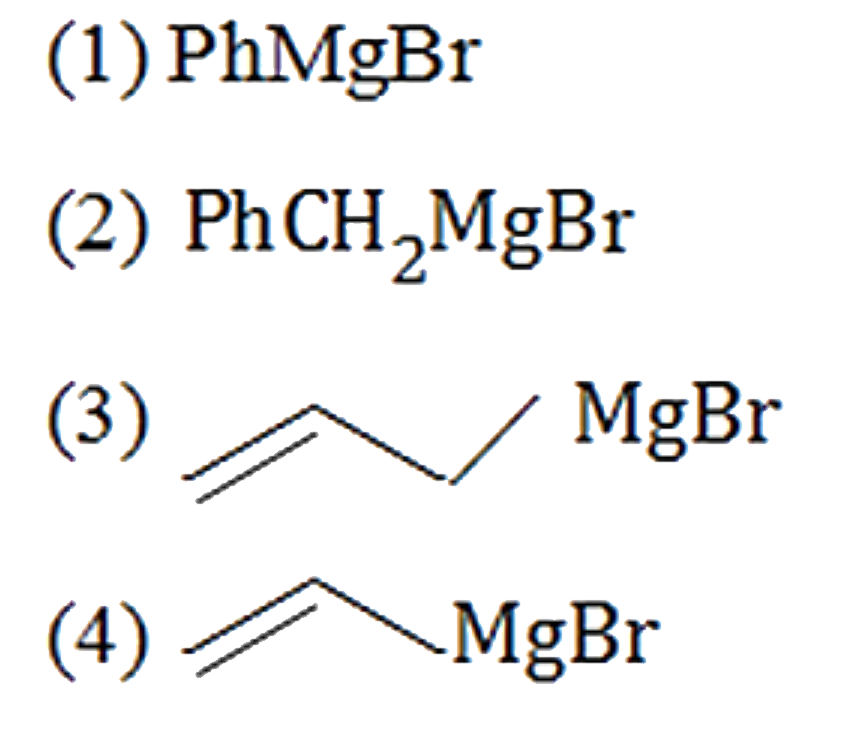
View SolutionArrange the following groups in decreasing activating order :
(i) −NR2
(ii) −Me
(iii) NHAc
(iv) −OH
View SolutionArrange the following in their decreasing order of acidity.
(I)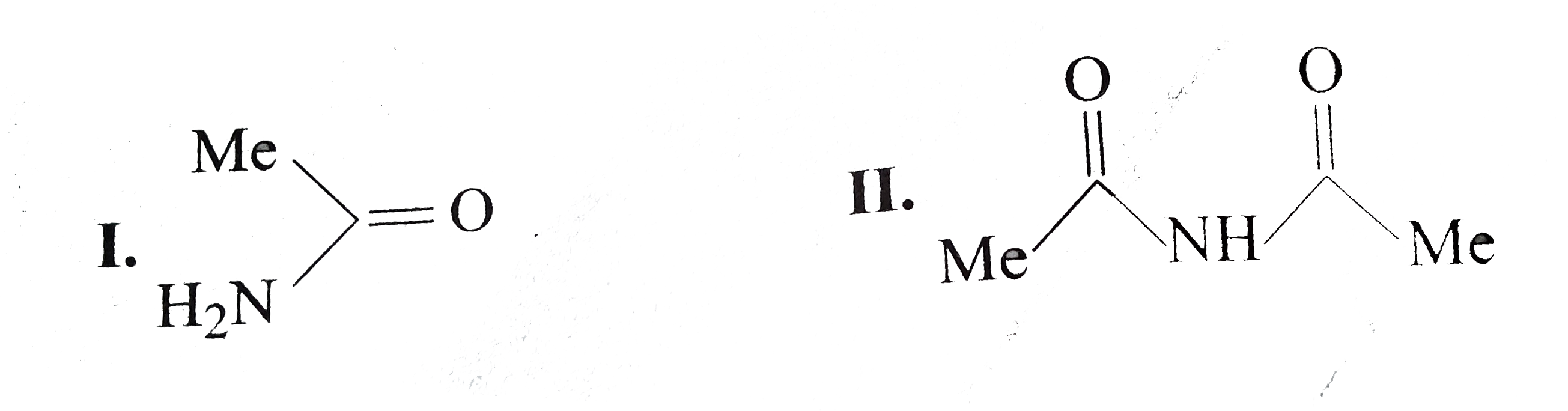
(III)
(IV) NH3.
View SolutionWhich of the following decreasing order of acid strength of
(i) Methanoic acid (ii) Ethanoic acid
(iii) Propanoic acid (iv) Butanoic acid
is correct ?
View Solution

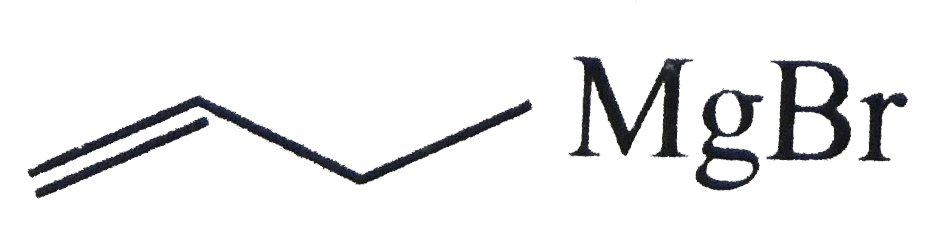
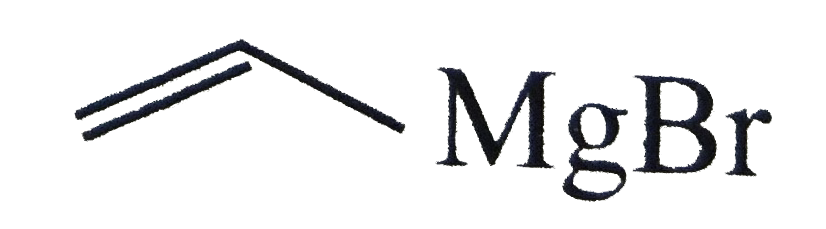 .
.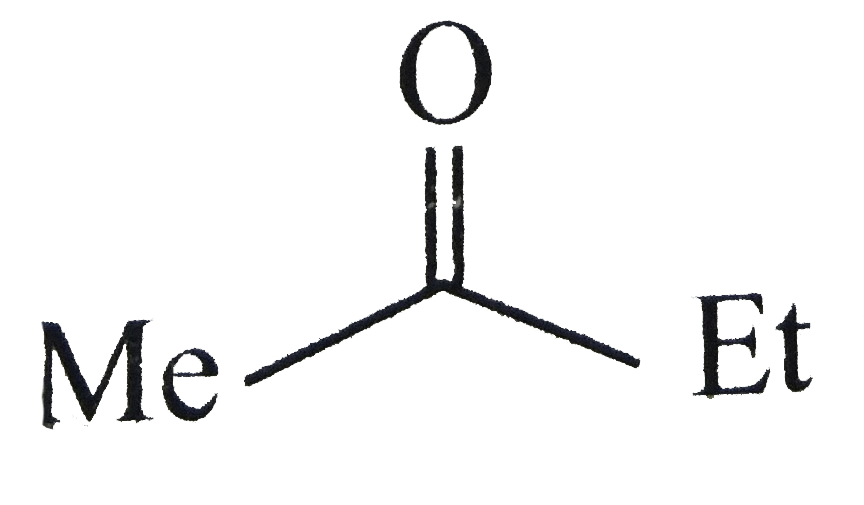 with the following
with the following